Unlock the Secrets: Electromagnetic Radiation EXPLAINED!

Electromagnetic radiation, a fundamental aspect of physics, is intrinsically linked to various phenomena, which the National Aeronautics and Space Administration (NASA) continuously studies to understand the universe. Frequency of electromagnetic radiation, inversely proportional to its wavelength, dictates its energy level. The spectrum of electromagnetic radiation lowest to highest energy ranges from radio waves used in communications technologies to gamma rays utilized in medical imaging; understanding this spectrum is essential. The Planck constant, a cornerstone of quantum mechanics, quantifies the relationship between a photon's energy and its frequency, providing a framework to analyze and classify electromagnetic radiation.
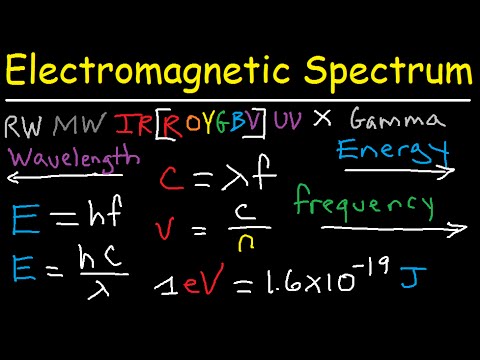
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light .
Electromagnetic radiation (EMR) is a fundamental force that permeates our universe, shaping everything from the warmth of the sun to the signals that power our smartphones. Though largely invisible to the human eye, EMR is an omnipresent and critical component of our existence. Understanding its nature and behavior is crucial to comprehending the world around us and harnessing its potential.
Defining Electromagnetic Radiation
Electromagnetic radiation is a form of energy that travels through space in the form of waves.
These waves are created by the acceleration of charged particles and consist of oscillating electric and magnetic fields that are perpendicular to each other and to the direction of propagation.
This radiation manifests in a spectrum of frequencies and wavelengths, each with unique properties and applications.
EMR is not merely an abstract scientific concept; it is the very fabric of light, heat, and wireless communication.
The Significance of Understanding the Electromagnetic Spectrum
The electromagnetic spectrum encompasses a vast range of radiation types, from low-frequency radio waves to high-frequency gamma rays.
Each segment of the spectrum has unique properties and interacts with matter in different ways, giving rise to a multitude of applications.
Understanding the electromagnetic spectrum is essential for numerous fields, including:
- Medicine: Enabling diagnostic imaging techniques like X-rays and MRI.
- Telecommunications: Facilitating wireless communication via radio waves and microwaves.
- Astronomy: Allowing us to study distant celestial objects by analyzing the electromagnetic radiation they emit.
- Environmental Science: Monitoring climate change and studying atmospheric phenomena.
Thesis Statement
This exploration of the electromagnetic spectrum, from radio waves to gamma rays, will elucidate the fundamental properties of EMR and its broad range of applications. By understanding the characteristics and behavior of each type of radiation, we can gain a deeper appreciation for its role in shaping our world and its potential to drive future technological advancements. We will uncover the properties, applications, and impact of this invisible force that shapes our world.
Electromagnetic radiation (EMR) stands as a testament to the intricacies of the universe, and grasping its essence requires understanding its fundamental properties. We've established that EMR is not just an abstract concept but a tangible force with diverse applications. To truly appreciate its potential and limitations, it's crucial to delve into the core characteristics that define its behavior.

The Nature of Electromagnetic Radiation: Waves and Particles
Electromagnetic radiation exhibits a fascinating duality, behaving as both a wave and a particle. This wave-particle duality is a cornerstone of quantum mechanics and is essential for understanding how EMR interacts with matter. It's not a matter of EMR being either a wave or a particle, but rather, it possesses properties of both, depending on how it's observed and measured.
The Wave Nature of Electromagnetic Radiation
As a wave, EMR is characterized by its oscillating electric and magnetic fields.
These fields propagate through space, transporting energy.
Key properties that describe its wave-like behavior include:
- Frequency (ν): The number of wave cycles that pass a given point per unit of time, typically measured in Hertz (Hz).
- Wavelength (λ): The distance between two consecutive crests or troughs of the wave, typically measured in meters.
The speed of light (c), a fundamental constant, relates these two properties:
c = λν
This equation highlights the inverse relationship between frequency and wavelength: higher frequency means shorter wavelength, and vice versa.
The Particle Nature of Electromagnetic Radiation
The particle nature of EMR is revealed through the concept of photons.
Photons are discrete packets of energy, often described as "quanta" of electromagnetic radiation.
Each photon carries a specific amount of energy that is directly proportional to the frequency of the radiation:
E = hν
Where:
- E is the energy of the photon.
- h is Planck's constant (approximately 6.626 x 10-34 joule-seconds).
- ν is the frequency of the radiation.
This equation is a cornerstone of quantum mechanics, demonstrating that energy is not continuous but rather quantized into discrete packets.
The Interplay of Frequency, Wavelength, and Energy
The equations c = λν and E = hν reveal the intricate relationships between frequency, wavelength, and energy.
A higher frequency corresponds to a shorter wavelength and a higher energy photon.
Conversely, a lower frequency corresponds to a longer wavelength and a lower energy photon.
This relationship explains why different types of electromagnetic radiation have vastly different effects on matter. For example, high-energy gamma rays can damage biological molecules, while low-energy radio waves are harmless to living organisms. Understanding these relationships is crucial for harnessing the power of EMR while mitigating its potential risks.
The Electromagnetic Spectrum: An Overview
Having explored the wave-particle duality that governs electromagnetic radiation, it's time to survey the landscape it inhabits: the electromagnetic spectrum. This spectrum is not merely a classification system; it's a map of the universe's energy, revealing the profound connections between different forms of radiation and their varied interactions with the world around us.
The electromagnetic spectrum is a continuum of all possible electromagnetic radiation frequencies. Arranged in order of increasing frequency (and therefore, decreasing wavelength and increasing energy), it encompasses a wide range of radiation types, each with unique properties and applications.
Exploring the Electromagnetic Spectrum's Regions
From the long wavelengths of radio waves to the incredibly short wavelengths of gamma rays, the electromagnetic spectrum is a diverse collection. Understanding its components is key to understanding the role of EMR in our lives.
-
Radio Waves: These are at the low-frequency, long-wavelength end of the spectrum.
-
Microwaves: These have shorter wavelengths and higher frequencies than radio waves.
-
Infrared Radiation: Often associated with heat, infrared radiation sits between microwaves and visible light.
-
Visible Light: This narrow band is the only part of the spectrum visible to the human eye.
-
Ultraviolet Radiation: Beyond visible light, ultraviolet radiation carries more energy.
-
X-rays: Known for their ability to penetrate soft tissues, X-rays are used extensively in medical imaging.
-
Gamma Rays: At the high-frequency, short-wavelength end, gamma rays are the most energetic form of electromagnetic radiation.
Organization: Energy, Frequency, and Wavelength
The arrangement of the electromagnetic spectrum isn't arbitrary. It's fundamentally organized by the relationship between energy, frequency, and wavelength. These three properties are inextricably linked.
A higher frequency means a shorter wavelength and higher energy, and vice versa. This inverse relationship between frequency and wavelength, governed by the constant speed of light, is essential to grasping how different parts of the spectrum interact with matter.
For example, radio waves, with their low frequencies, are used for long-distance communication because they can travel long distances. Gamma rays, with their high frequencies and energy, are used in cancer treatment but also pose a significant radiation hazard.
Visualizing the Spectrum
While we can't "see" the entire electromagnetic spectrum, a visual representation can greatly aid understanding. Diagrams typically depict the spectrum as a horizontal band, with the different types of radiation arranged from left to right based on their frequency and wavelength.
These diagrams often include:
- The relative sizes of the wavelengths.
- Examples of common sources and applications for each type of radiation.
- A scale showing frequency or wavelength values.
Such visuals help to solidify the concept of the spectrum as a continuous range, rather than just a collection of disparate types of radiation. They also emphasize the vast differences in energy and wavelength that exist across the spectrum.
Having surveyed the vast expanse of the electromagnetic spectrum, we now turn our attention to its lower end, where the longest wavelengths and lowest frequencies reside: radio waves. These often-overlooked waves are not merely a footnote in the spectrum but rather a cornerstone of modern communication and technology, silently facilitating countless interactions that shape our daily lives.
Radio Waves: Communication and Beyond
Radio waves, characterized by their long wavelengths and correspondingly low frequencies, occupy the portion of the electromagnetic spectrum below approximately 300 GHz. This region is far from empty; it's a bustling hub of activity, carrying the signals that connect us to the world.
Defining Characteristics
Unlike their higher-energy counterparts, radio waves possess wavelengths that can range from millimeters to hundreds of kilometers.
This vast range in wavelength allows for a variety of applications, each tailored to specific frequency bands.
Their low frequency also means that radio waves carry relatively little energy individually, making them generally non-ionizing and considered safe for everyday exposure.
Telecommunications Backbone
The primary application of radio waves lies in telecommunications.
From the crackling voice on an AM radio to the high-definition video streaming on your smartphone, radio waves are the invisible carriers of information.
Radio broadcasting utilizes radio waves to transmit audio signals across vast distances, bringing news, music, and entertainment to listeners worldwide.
Television broadcasting similarly relies on radio waves to transmit both audio and video signals, enabling viewers to receive programming over the air.
Mobile phone technology is perhaps the most ubiquitous example of radio wave communication.
Cellular networks utilize a complex system of base stations and radio frequencies to allow users to make calls, send texts, and access the internet wirelessly.
Beyond Communication: Diverse Applications
While telecommunications dominate the use of radio waves, their applications extend far beyond this realm.
Radar Technology
Radar, or Radio Detection and Ranging, utilizes radio waves to detect the presence, direction, distance, and speed of objects.
By emitting radio waves and analyzing the reflected signals, radar systems can provide valuable information in a variety of settings.
These settings include air traffic control, weather forecasting, and law enforcement.
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI), a powerful medical imaging technique, leverages the properties of radio waves in conjunction with strong magnetic fields.
While the underlying principles are complex, MRI essentially uses radio waves to excite hydrogen atoms in the body, generating signals that can be used to create detailed images of internal organs and tissues.
The reason for MRI's inclusion here, despite its complexities, is to showcase the surprising range of radio wave applications and how the same electromagnetic radiation used for simple communications can be harnessed for advanced medical diagnostics.
Other Applications
Radio waves also play a role in various other technologies, including:
- Amateur Radio: Allowing hobbyists to communicate across long distances.
- Satellite Communication: Enabling global communication and broadcasting.
- Remote Control Systems: Used in everything from garage door openers to drones.
Radio waves, though often unseen and unappreciated, are an indispensable part of our modern world. Their unique properties make them ideally suited for a wide range of applications, from broadcasting and telecommunications to medical imaging and radar technology. As technology continues to evolve, radio waves will undoubtedly continue to play a vital role in shaping the way we communicate, navigate, and understand the world around us.
Having surveyed the vast expanse of the electromagnetic spectrum, we now turn our attention to its lower end, where the longest wavelengths and lowest frequencies reside: radio waves. These often-overlooked waves are not merely a footnote in the spectrum but rather a cornerstone of modern communication and technology, silently facilitating countless interactions that shape our daily lives.
Building upon the understanding of radio waves and their fundamental role, let's ascend slightly higher in frequency and delve into another pivotal region of the electromagnetic spectrum: microwaves. These waves, shorter than radio waves but longer than infrared radiation, possess unique characteristics that make them indispensable in a variety of applications, from heating our food to connecting us globally.
Microwaves: Cooking, Communication, and More
Microwaves occupy a significant portion of the electromagnetic spectrum, bridging the gap between radio waves and infrared radiation. Their unique properties enable a diverse range of applications that profoundly impact our daily lives. From the convenience of rapid cooking to the sophistication of global communication, microwaves are a testament to the power of harnessing electromagnetic energy.
Understanding Microwaves: Properties and Interactions
Microwaves, with wavelengths typically ranging from millimeters to meters, exhibit behavior distinct from other forms of electromagnetic radiation. Their interaction with matter, particularly water molecules, is key to many of their applications.
Microwaves cause polar molecules, like water, to vibrate rapidly. This vibration generates heat, which is the basis for microwave oven technology.
Unlike ionizing radiation, microwaves are non-ionizing. They do not possess enough energy to remove electrons from atoms. This makes them generally safe for everyday use when properly contained.
Microwaves in the Kitchen: The Science of Microwave Ovens
The most familiar application of microwaves is undoubtedly the microwave oven. These ubiquitous appliances leverage the unique properties of microwaves to provide rapid and efficient heating.
How Microwave Ovens Work
Microwave ovens generate microwaves at a specific frequency (typically 2.45 GHz). This frequency is particularly effective at causing water molecules to vibrate.
The microwaves are distributed within the oven cavity, causing water molecules in the food to vibrate. This generates heat throughout the food.
The metal mesh on the oven door acts as a Faraday cage, preventing microwaves from escaping and ensuring safe operation. This containment is crucial for preventing harmful exposure.
Advantages and Limitations
Microwave ovens offer unparalleled speed and convenience in food preparation. They are particularly effective for reheating pre-cooked meals.
However, microwave heating can be uneven. Foods with high water content tend to heat faster.
Additionally, some materials, such as metal, can reflect microwaves, leading to sparking and potential damage to the oven.
Beyond Cooking: Microwaves in Communication and Technology
Beyond their culinary applications, microwaves play a vital role in various communication and technological systems. Their relatively short wavelengths allow for efficient transmission and reception of signals.
Satellite Communication
Microwaves are extensively used in satellite communication. This is due to their ability to penetrate the Earth's atmosphere with minimal interference.
Satellites in geostationary orbit transmit and receive microwave signals. This relays data, voice, and video across vast distances.
Radar Systems
Radar (Radio Detection and Ranging) systems rely on microwaves to detect and track objects. These systems emit microwave pulses and analyze the reflected signals.
The time it takes for the signal to return provides information about the distance and location of the object.
Radar is used in a wide range of applications, including weather forecasting, air traffic control, and military surveillance.
Other Applications
Microwaves are also used in various other applications, including:
- Wireless networking (Wi-Fi): Enables high-speed internet access without cables.
- Medical treatments: Used in some therapeutic applications.
- Industrial processes: For drying, sterilization, and material processing.
Microwaves, therefore, are not merely the energy source behind a convenient cooking appliance. They represent a versatile and essential component of modern technology, facilitating communication, navigation, and a host of other critical functions.
Building upon the understanding of radio waves and their fundamental role, let's ascend slightly higher in frequency and delve into another pivotal region of the electromagnetic spectrum: microwaves. These waves, shorter than radio waves but longer than infrared radiation, possess unique characteristics that make them indispensable in a variety of applications, from heating our food to connecting us globally. Moving along the spectrum, we encounter infrared radiation, an area less about communication in the traditional sense and more about sensing the world through heat.
Infrared Radiation: Heat and Imaging
Infrared (IR) radiation occupies the portion of the electromagnetic spectrum between microwaves and visible light. Often associated with heat, infrared radiation plays a critical role in a surprisingly wide array of technologies and natural phenomena.
Infrared Radiation as Heat
At its core, infrared radiation is felt as heat. This is because infrared waves cause molecules to vibrate more rapidly, increasing their thermal energy.
Everything with a temperature above absolute zero emits infrared radiation. The hotter an object, the more infrared radiation it emits.
This fundamental principle explains why we feel the warmth of the sun, a fire, or a hot stovetop, even without direct contact.
Thermal Imaging: Seeing the Invisible
Thermal imaging, also known as thermography, is a technique that utilizes infrared radiation to create images based on temperature differences. Specialized cameras detect the infrared radiation emitted by objects and translate it into a visible image.
Applications of Thermal Imaging
Thermal imaging has numerous applications across diverse fields:
-
Building Inspection: Detecting heat loss and insulation deficiencies in buildings.
-
Medical Diagnostics: Identifying areas of inflammation or abnormal blood flow in the human body.
-
Law Enforcement: Locating suspects in darkness or through smoke, and detecting illegal activities like indoor marijuana cultivation.
-
Industrial Maintenance: Identifying overheating components in machinery before they fail.
-
Search and Rescue: Finding people in disaster zones or during nighttime searches.
Thermal imaging provides a non-invasive and contactless way to "see" temperature variations, offering valuable insights in situations where traditional visual inspection is insufficient.
Everyday Applications of Infrared Technology
Beyond thermal imaging, infrared technology has become commonplace in our daily lives:
-
Remote Controls: Most remote controls for televisions, stereos, and other electronic devices use infrared signals to transmit commands. The remote emits a coded infrared signal that is detected by the device.
-
Security Systems: Infrared motion detectors are used in security systems to detect movement by sensing changes in infrared radiation. When a person enters the field of view, their body heat triggers the alarm.
-
Night Vision Technology: Night vision goggles and cameras amplify the available infrared light, allowing us to see in the dark. This technology is widely used by the military, law enforcement, and for surveillance purposes.
These examples demonstrate the ubiquity of infrared radiation in technologies that enhance our convenience, safety, and security.
Visible Light: The Colors We See
Having journeyed through the realms of infrared radiation, where heat reigns supreme, we now arrive at a truly unique and captivating portion of the electromagnetic spectrum: visible light. This narrow band, nestled between infrared and ultraviolet radiation, holds a special significance for us. It is, quite simply, the only part of the spectrum directly perceptible to the human eye.
The Eye's Window to the World
Visible light, often referred to as the optical spectrum, is the range of electromagnetic radiation that our eyes can detect. It's the key to our visual perception, allowing us to experience the world in a vibrant tapestry of colors, shapes, and forms.
Without it, our world would be shrouded in perpetual darkness, and our understanding of the universe would be significantly limited.
A Spectrum of Colors
Within the realm of visible light lies a spectrum of colors, each corresponding to a specific wavelength and frequency. When white light, such as sunlight, passes through a prism, it is dispersed into its constituent colors: red, orange, yellow, green, blue, indigo, and violet. This is the iconic rainbow effect.
Each color represents a different wavelength of light. Red has the longest wavelength, and violet has the shortest. Our perception of color arises from the way our eyes and brains interpret these different wavelengths.
How We See Color
The human eye contains specialized cells called photoreceptors in the retina: rods and cones. Rods are responsible for black and white vision in low light conditions. Cones, on the other hand, are responsible for color vision and function best in bright light.
There are three types of cones, each sensitive to a different range of wavelengths: red, green, and blue. Our brains interpret the relative stimulation of these cones to perceive the full spectrum of colors.
Color blindness occurs when one or more types of cones are defective or missing, leading to an altered perception of color.
Photosynthesis: Light as Life
Beyond its role in human vision, visible light plays an indispensable role in the natural world, most notably in photosynthesis. This is the process by which plants, algae, and some bacteria convert light energy into chemical energy in the form of sugars.
Chlorophyll, the green pigment found in plants, absorbs sunlight, primarily in the blue and red regions of the spectrum. This absorbed light energy fuels the conversion of carbon dioxide and water into glucose (sugar) and oxygen.
Photosynthesis is the foundation of most food chains on Earth, providing the energy and oxygen necessary for the survival of countless organisms, including ourselves. Without visible light, photosynthesis would cease, and life as we know it would be unsustainable.
Visible light, therefore, is far more than just a source of illumination. It is a fundamental component of our perception, our planet's ecosystems, and ultimately, our very existence.
Having explored the mesmerizing realm of visible light, where color dances and photosynthesis thrives, we now transition to a region of the electromagnetic spectrum that is both essential for life and potentially hazardous: ultraviolet radiation. While invisible to the naked eye, its impact is undeniable, shaping our health, environment, and even technological advancements. Understanding the duality of ultraviolet radiation, its benefits and risks, is crucial in navigating our interaction with this potent form of energy.
Ultraviolet Radiation: A Double-Edged Sword
Ultraviolet (UV) radiation occupies the portion of the electromagnetic spectrum between visible light and X-rays. It carries more energy than visible light but less than X-rays, a factor that determines its unique effects on matter and living organisms. UV radiation is categorized into three bands: UVA, UVB, and UVC, each with distinct properties and varying degrees of penetration and biological impact. While largely invisible to the human eye, ultraviolet radiation plays a crucial role in our atmosphere and daily lives.
The Skin's Response to UV Exposure
The most noticeable effect of UV radiation is on the skin. Exposure to UV light triggers a cascade of biological processes. Tanning, often considered a desirable cosmetic effect, is actually the skin's defense mechanism against UV damage. Melanocytes, specialized cells in the skin, produce melanin, a pigment that absorbs UV radiation and darkens the skin. This increased pigmentation helps to protect underlying cells from further damage.
However, excessive UV exposure can overwhelm the skin's protective mechanisms, leading to sunburn. Sunburn is an inflammatory response caused by damage to DNA in skin cells. It manifests as redness, pain, and, in severe cases, blistering. Repeated or intense sunburns significantly increase the risk of developing skin cancer later in life.
Skin cancer, including basal cell carcinoma, squamous cell carcinoma, and melanoma, is a serious consequence of prolonged UV exposure. UV radiation damages the DNA in skin cells, leading to uncontrolled growth and the formation of cancerous tumors. Melanoma, the most dangerous form of skin cancer, can spread rapidly to other parts of the body, making early detection and treatment crucial.
Atmospheric Absorption of UV Radiation
The Earth's atmosphere plays a vital role in filtering out harmful UV radiation, protecting life on the planet. The ozone layer, located in the stratosphere, is particularly effective at absorbing UVC and UVB radiation. Ozone molecules (O3) absorb UV photons, breaking down into oxygen molecules (O2) and single oxygen atoms (O). These then recombine to form ozone again, continuously absorbing UV radiation in the process.
Unfortunately, human activities have led to the depletion of the ozone layer, particularly through the release of chlorofluorocarbons (CFCs) and other ozone-depleting substances. This depletion allows more harmful UV radiation to reach the Earth's surface, increasing the risk of skin cancer and other adverse health effects. International efforts, such as the Montreal Protocol, have been successful in phasing out many ozone-depleting substances, leading to a slow but steady recovery of the ozone layer.
Benefits and Applications of UV Radiation
Despite its potential dangers, UV radiation also has beneficial applications. UVB radiation is essential for vitamin D production in the skin. When UVB photons strike the skin, they convert a precursor molecule into vitamin D3, which is then processed by the liver and kidneys into active vitamin D. Vitamin D is crucial for calcium absorption, bone health, and immune function.
UV radiation is also used for sterilization and disinfection. UVC radiation, in particular, is highly effective at killing bacteria, viruses, and other microorganisms. It is used in hospitals, water treatment plants, and air purification systems to prevent the spread of infectious diseases. UV sterilization is also employed in food processing and packaging to extend shelf life and ensure food safety.
Tanning beds utilize UVA and UVB radiation to artificially tan the skin. While tanning beds may provide a cosmetic tan, they also significantly increase the risk of skin cancer. The World Health Organization (WHO) and other health organizations strongly advise against the use of tanning beds due to their associated health risks. The perceived aesthetic benefits do not outweigh the scientifically proven dangers.
Understanding the multifaceted nature of UV radiation – its capacity to both harm and heal – is key to mitigating its risks and harnessing its potential benefits. Protecting ourselves from excessive exposure, while utilizing its beneficial applications, allows us to navigate this part of the electromagnetic spectrum safely and responsibly.
Having explored the mesmerizing realm of visible light, where color dances and photosynthesis thrives, we now transition to a region of the electromagnetic spectrum that is both essential for life and potentially hazardous: ultraviolet radiation. While invisible to the naked eye, its impact is undeniable, shaping our health, environment, and even technological advancements. Understanding the duality of ultraviolet radiation, its benefits and risks, is crucial in navigating our interaction with this potent form of energy.
X-rays: Penetrating Power and Medical Imaging
X-rays represent a significant step up the electromagnetic spectrum in terms of energy and, consequently, penetrating power. This characteristic, born from their shorter wavelengths, makes them invaluable in a multitude of applications, most notably in medical imaging, where they allow us to visualize the inner workings of the human body. However, this very same power also necessitates a careful understanding of their potential risks.
The Nature of X-ray Penetration
The defining characteristic of X-rays is their ability to penetrate materials that are opaque to visible light. This arises from their high energy and short wavelength, allowing them to pass through soft tissues relatively easily, while being absorbed more readily by denser materials like bone and metal.
This differential absorption is the key to X-ray imaging.
The degree of penetration is directly related to the energy of the X-ray photons. Higher energy X-rays are more penetrating, while lower energy X-rays are more readily absorbed. This principle is carefully controlled in practical applications to optimize image quality and minimize patient exposure.
Medical Radiography: A Window into the Body
Medical radiography, commonly known as X-ray imaging, is a cornerstone of modern diagnostics. It utilizes the differential absorption of X-rays to create images of bones, organs, and other internal structures.
The Process of Radiography
In a typical X-ray procedure, a patient is positioned between an X-ray source and a detector. The X-rays pass through the body, and the detector captures the pattern of absorption. This pattern is then converted into an image, where denser tissues appear whiter (due to greater absorption) and less dense tissues appear darker.
Applications in Diagnostics
X-rays are used to diagnose a wide range of conditions, including:
-
Bone fractures and dislocations: X-rays are highly effective at visualizing bone structures, making them essential for diagnosing fractures and dislocations.
-
Pneumonia and other lung conditions: X-rays can reveal abnormalities in the lungs, such as infections or tumors.
-
Dental problems: Dental X-rays are used to detect cavities, impacted teeth, and other dental issues.
-
Foreign objects: X-rays can be used to locate foreign objects that may have been swallowed or lodged in the body.
Beyond Medicine: Security and Industrial Applications
The penetrating power of X-rays extends their usefulness beyond the medical field. They are employed in security and industrial settings for non-destructive testing and inspection.
Airport Security Scanners
Airport security scanners utilize X-rays to screen luggage and cargo for prohibited items. These scanners generate images that reveal the contents of bags without requiring them to be opened, enhancing security measures.
Industrial Radiography
In industry, X-rays are used to inspect welds, castings, and other manufactured components for defects. This technique, known as industrial radiography, helps to ensure the quality and safety of products in various sectors, from aerospace to construction.
By detecting internal flaws that are invisible to the naked eye, X-ray inspection prevents potential failures and enhances the reliability of critical infrastructure.
The versatility of X-rays, stemming from their unique ability to penetrate matter, solidifies their importance in medicine, security, and industry. However, responsible usage and stringent safety protocols remain paramount, due to their ionizing nature and potential health risks with prolonged exposure.
Gamma Rays: Harnessing High-Energy Radiation for Good, While Mitigating the Risks
Having explored the mesmerizing realm of visible light, where color dances and photosynthesis thrives, we now transition to a region of the electromagnetic spectrum that is both essential for life and potentially hazardous: ultraviolet radiation. While invisible to the naked eye, its impact is undeniable, shaping our health, environment, and even technological advancements. Understanding the duality of ultraviolet radiation, its benefits and risks, is crucial in navigating our interaction with this potent form of energy.
X-rays, with their capacity to pierce through surfaces, lead us to another section of the spectrum. This brings us to its most extreme end: gamma rays. These represent the highest-energy form of electromagnetic radiation. They possess a unique set of characteristics, stemming from their extremely short wavelengths and correspondingly high frequencies. These characteristics dictate their origin, applications, and, most importantly, the precautions necessary when dealing with them.
The Nature and Origins of Gamma Rays
Gamma rays are not produced by simple processes. Unlike radio waves generated by oscillating circuits or visible light emitted by heated objects, gamma rays originate from the most energetic phenomena in the universe.
Radioactive Decay
One of the primary sources is radioactive decay. Certain unstable atomic nuclei release energy in the form of gamma rays. This occurs when a nucleus transitions from a high-energy state to a more stable state. The emitted gamma rays carry away the excess energy.
Cosmic Events
Beyond Earth, colossal cosmic events like supernovae and black hole accretion disks produce gamma rays. These cataclysmic events unleash enormous amounts of energy. Some of this energy manifests as intense bursts of gamma radiation.
Applications of Gamma Rays
Despite their potential danger, gamma rays have proven invaluable across various fields. Their high energy, if managed carefully, can be harnessed for the betterment of society.
Cancer Treatment: Radiation Therapy
One of the most significant applications is in radiation therapy for cancer treatment. Focused beams of gamma rays can target and destroy cancerous cells. This treatment damages their DNA and prevents them from multiplying. This controlled destruction helps to shrink tumors and eradicate the disease.
The precision of modern radiation therapy techniques aims to minimize damage to surrounding healthy tissues.
Industrial Uses
Gamma rays also find use in industrial radiography. Similar to how X-rays are used in medicine, gamma rays can inspect the integrity of materials and structures.
This is vital in industries like aerospace and construction. It enables the detection of flaws and weaknesses that would otherwise be invisible. Flaws like these could compromise safety and reliability. They are also used for sterilization of medical equipment, by killing bacteria and viruses.
The Risks of Gamma Radiation Exposure
It is crucial to remember that the very properties that make gamma rays useful also make them potentially harmful. Their high energy allows them to penetrate deep into matter, including living tissue.
Cellular Damage and Health Risks
When gamma rays interact with cells, they can damage DNA. This damage can lead to mutations. These mutations can then increase the risk of cancer. High doses of gamma radiation can also cause acute radiation sickness. This can result in symptoms ranging from nausea and fatigue to more severe conditions. Severe conditions include organ failure and even death.
Safety Precautions
Due to these risks, strict safety protocols are essential when working with gamma radiation sources. These measures include:
-
Shielding: Using dense materials like lead or concrete to absorb gamma rays.
-
Distance: Maximizing the distance from the source to reduce exposure intensity.
-
Time Limitation: Minimizing the duration of exposure.
-
Monitoring: Employing radiation detectors to monitor exposure levels and ensure safety.
Understanding the nature and properties of gamma rays. Understanding both their potential benefits and risks is essential. It allows us to harness their power responsibly. It also mitigates any potential danger. Continued research and development in radiation safety are crucial. This ensures the continued safe use of gamma rays for the benefit of society.
Having explored the potential hazards and remarkable utility of gamma rays, it's crucial to step back and consider a broader categorization of electromagnetic radiation based on its capacity to alter the very structure of matter it encounters. This leads us to the critical distinction between ionizing and non-ionizing radiation – a difference that dictates the level of risk and the precautions necessary when interacting with these invisible forces.
Ionizing vs. Non-ionizing Radiation: Understanding the Dangers
Electromagnetic radiation is not all created equal. While all forms of EMR carry energy, only some possess enough to dislodge electrons from atoms, a process known as ionization. This ability to ionize matter is the key differentiator between ionizing and non-ionizing radiation. Understanding this distinction is paramount to assessing potential hazards and implementing appropriate safety measures.
Ionizing Radiation: The High-Energy Threat
Ionizing radiation possesses sufficient energy to remove electrons from atoms and molecules, creating ions. This process can disrupt chemical bonds and damage biological tissues, making it a significant health hazard.
Examples of ionizing radiation include:
- X-rays
- Gamma rays
- High-energy ultraviolet (UV) radiation
Dangers of Ionizing Radiation
The primary danger of ionizing radiation lies in its ability to damage DNA, the fundamental building block of life. This damage can lead to:
- Cellular mutations
- Cell death
- An increased risk of cancer
The severity of the damage depends on factors like:
- The dose of radiation
- The duration of exposure
- The type of tissue exposed
Even low doses of ionizing radiation can pose a long-term risk, emphasizing the need for careful control and mitigation strategies. Certain organs, such as the bone marrow and thyroid, are particularly vulnerable to the effects of ionizing radiation.
Non-ionizing Radiation: A Lower-Energy Landscape
Non-ionizing radiation, on the other hand, lacks the energy to remove electrons from atoms. Instead, it typically causes atoms and molecules to vibrate or heat up. While generally considered less harmful than ionizing radiation, certain types can still pose potential health risks.
Examples of non-ionizing radiation include:
- Radio waves
- Microwaves
- Infrared radiation
- Visible light
- Low-energy ultraviolet (UV) radiation
Safety and Potential Long-Term Effects
Non-ionizing radiation is generally considered safe at typical exposure levels. However, high-intensity exposure can still cause harm. For example, intense infrared radiation can cause burns, and prolonged exposure to blue light from electronic devices may affect sleep patterns.
The long-term effects of exposure to non-ionizing radiation are a subject of ongoing research. Some studies have suggested potential links between long-term exposure to certain types of non-ionizing radiation, such as radiofrequency fields from mobile phones, and an increased risk of certain cancers. However, these links are not definitively proven, and more research is needed to fully understand the potential risks.
Prudent Avoidance
Even though the dangers of non-ionizing radiation may be less immediate and certain, adopting a principle of prudent avoidance is advisable. This means taking reasonable steps to minimize exposure, especially prolonged or high-intensity exposure, to non-ionizing radiation sources. This could include:
- Using hands-free devices for mobile phones
- Limiting screen time
- Maintaining a safe distance from high-power radiofrequency sources.
By understanding the fundamental differences between ionizing and non-ionizing radiation, we can make informed decisions about our exposure and take appropriate precautions to protect our health and well-being. While ionizing radiation poses a more direct and immediate threat, a mindful approach to non-ionizing radiation is also warranted, especially in our increasingly technology-rich environment.
Having explored the potential hazards and remarkable utility of gamma rays, it's crucial to step back and consider a broader categorization of electromagnetic radiation based on its capacity to alter the very structure of matter it encounters. This leads us to the critical distinction between ionizing and non-ionizing radiation – a difference that dictates the level of risk and the precautions necessary when interacting with these invisible forces.
Applications and Impact: Electromagnetic Radiation in Our World
Electromagnetic radiation (EMR) isn't just an abstract scientific concept. It's a driving force behind countless technologies that shape our modern lives. From the doctor's office to the devices in our pockets, EMR plays a crucial, often unseen, role. Its versatility makes it an indispensable tool across diverse fields.
Medical Marvels: EMR in Healthcare
Medical imaging has been revolutionized by the application of different types of EMR. X-rays, with their penetrating power, allow doctors to visualize bone structures and detect abnormalities. Traditional radiography remains a cornerstone of diagnostic medicine.
However, it is important to remember that while x-rays have greatly benefitted the field of medical imaging, these processes are not without risk.
Magnetic Resonance Imaging (MRI) offers a complementary view. It allows for detailed imaging of soft tissues, and organs without the use of ionizing radiation. MRI relies on radio waves and strong magnetic fields to generate images. This makes it a safer alternative for certain applications.
Telecommunications: Connecting the Globe
Our interconnected world is built upon the invisible infrastructure of electromagnetic waves. Radio waves and microwaves are the workhorses of modern telecommunications. They enable everything from broadcasting to mobile communication.
Radio waves transmit audio and video signals across vast distances. They power radio stations, television networks, and countless other services. Microwaves facilitate satellite communication, connecting remote locations and enabling global connectivity.
The ubiquitous mobile phone relies on both radio waves and microwaves to function. These waves transmit voice and data between devices and cellular towers. They are crucial for the seamless communication we often take for granted.
Beyond the Obvious: Diverse Applications of EMR
The applications of EMR extend far beyond medical imaging and telecommunications. They permeate various industrial processes, scientific research endeavors, and consumer products. This includes applications such as:
-
Industrial Processes: Infrared radiation is used for heating, drying, and curing in manufacturing. Lasers (focused beams of light) are used for precision cutting, welding, and marking.
-
Scientific Research: EMR is an essential tool for scientific research, where specialized tools can use EMR to detect, analyze, and view materials. Spectrometers analyze the composition of materials. Telescopes gather light from distant stars. Particle accelerators use radio waves to accelerate subatomic particles.
-
Consumer Products: From the infrared remote control to the UV sterilizers, EMR has found its way into many consumer products. LED lighting, based on visible light, offers energy-efficient illumination.
The impact of electromagnetic radiation on our world is undeniable. It continues to be a catalyst for innovation and advancement across various fields. From healthcare to telecommunications, EMR empowers us with technologies that improve our lives. As research progresses, we can expect even more exciting applications of this fundamental force.
Having explored the potential hazards and remarkable utility of gamma rays, it's crucial to step back and consider a broader categorization of electromagnetic radiation based on its capacity to alter the very structure of matter it encounters. This leads us to the critical distinction between ionizing and non-ionizing radiation – a difference that dictates the level of risk and the precautions necessary when interacting with these invisible forces.
Planck's Constant and the Quantum World of Energy
While understanding the spectrum and applications of electromagnetic radiation is vital, a deeper dive into the fundamental physics unveils the intricacies governing energy behavior at the quantum level. Planck's constant, a cornerstone of quantum mechanics, is indispensable for grasping this nuanced relationship between frequency and energy.
The Significance of Planck's Constant (h)
At the dawn of the 20th century, Max Planck revolutionized physics by introducing the concept of energy quantization. He proposed that energy is not emitted or absorbed continuously. Instead, it comes in discrete packets called quanta.
This groundbreaking idea hinged on a constant, now known as Planck's constant (h), which has a value of approximately 6.626 x 10-34 joule-seconds. This constant acts as the fundamental unit that links the energy of a photon to its frequency.
Planck's constant underpins numerous quantum phenomena, including the photoelectric effect and the behavior of blackbody radiation. Without it, much of modern physics and technology would be incomprehensible.
Energy Quanta: Discretizing the Continuous
Classical physics viewed energy as a continuous variable, capable of assuming any value. Planck's theory shattered this notion, asserting that energy is quantized, meaning it exists only in specific, discrete amounts.
Imagine energy as a staircase rather than a ramp. You can only stand on specific steps (quanta), not anywhere in between. Each quantum of energy is directly proportional to the frequency of the electromagnetic radiation.
This concept is particularly relevant when dealing with electromagnetic radiation, where energy is carried by photons. Each photon possesses a specific amount of energy determined by its frequency and Planck's constant.
E=hf: Unveiling the Relationship Between Energy and Frequency
The equation E=hf succinctly encapsulates the relationship between energy (E) and frequency (f) of electromagnetic radiation. Here, h represents Planck's constant, serving as the proportionality factor.
This simple equation reveals that the energy of a photon is directly proportional to its frequency. Higher frequency radiation (like X-rays and gamma rays) carries more energy per photon than lower frequency radiation (like radio waves).
This relationship has profound implications. It explains why high-frequency electromagnetic radiation is more likely to cause ionization and damage to living tissues. It also allows scientists and engineers to precisely control and manipulate electromagnetic radiation for various applications, from medical treatments to advanced communication technologies. The ability to calculate and predict the energy levels associated with different frequencies is crucial in diverse fields, reinforcing the importance of understanding Planck's constant and the quantized nature of energy.
Video: Unlock the Secrets: Electromagnetic Radiation EXPLAINED!
FAQs About Electromagnetic Radiation
Here are some frequently asked questions to clarify your understanding of electromagnetic radiation.
What exactly is electromagnetic radiation?
Electromagnetic radiation is a form of energy that travels through space as waves. It's produced when an electrically charged particle accelerates. It includes everything from the light we see to radio waves and X-rays.
What's the difference between radio waves and gamma rays?
The main difference is their energy and wavelength. Radio waves have the lowest energy and longest wavelengths, while gamma rays have the highest energy and shortest wavelengths. This difference determines how they interact with matter.
How does the energy of electromagnetic radiation affect its uses?
Higher energy electromagnetic radiation like X-rays and gamma rays can penetrate materials, which makes them useful for medical imaging and cancer treatment. Lower energy radiation, like radio waves, is used for communication because it travels long distances. The order of electromagnetic radiation lowest to highest energy dictates their various applications.
Can electromagnetic radiation be harmful?
Yes, high-energy electromagnetic radiation like UV, X-rays, and gamma rays can be harmful because they can damage cells and DNA. Lower energy radiation, like radio waves and microwaves, are generally considered safe at normal exposure levels.
Hopefully, you now have a better grasp of electromagnetic radiation, especially how it's organized from electromagnetic radiation lowest to highest energy! Keep exploring the amazing world around you!