Unlock Semiconductor Secrets: Valence vs. Conduction Bands!

Understanding semiconductor behavior is fundamental to modern electronics, impacting everything from your smartphone to advanced computing systems. At the heart of this understanding lies the concept of energy bands. The Energy Gap, a core concept in solid-state physics, dictates whether a material acts as a conductor, insulator, or semiconductor. Silicon (Si), the most common semiconductor material, owes its unique electrical properties to the specific arrangement and behavior of electrons within its valence and conduction bands. The properties of these bands are thoroughly investigated and manipulated in laboratories like the Microelectronics Research Center, driving innovation in device performance. Many advances in transistor technology are because of engineers learning about and controlling the flow of electron. Consequently, what is valence band and conduction band in semiconductor is a crucial question for anyone seeking to delve deeper into the intricacies of semiconductor physics and engineering.

Image taken from the YouTube channel Professor Dave Explains , from the video titled Conductivity and Semiconductors .
Unveiling Semiconductor Secrets: Valence vs. Conduction Bands
Understanding how semiconductors work requires diving into the core concepts of valence and conduction bands. These energy bands dictate a material's ability to conduct electricity, differentiating insulators, conductors, and the star of our show: semiconductors. Let's explore "what is valence band and conduction band in semiconductor" in detail.
Energy Bands: A Foundation
Before we focus specifically on semiconductors, it's important to understand the broader concept of energy bands within solids.
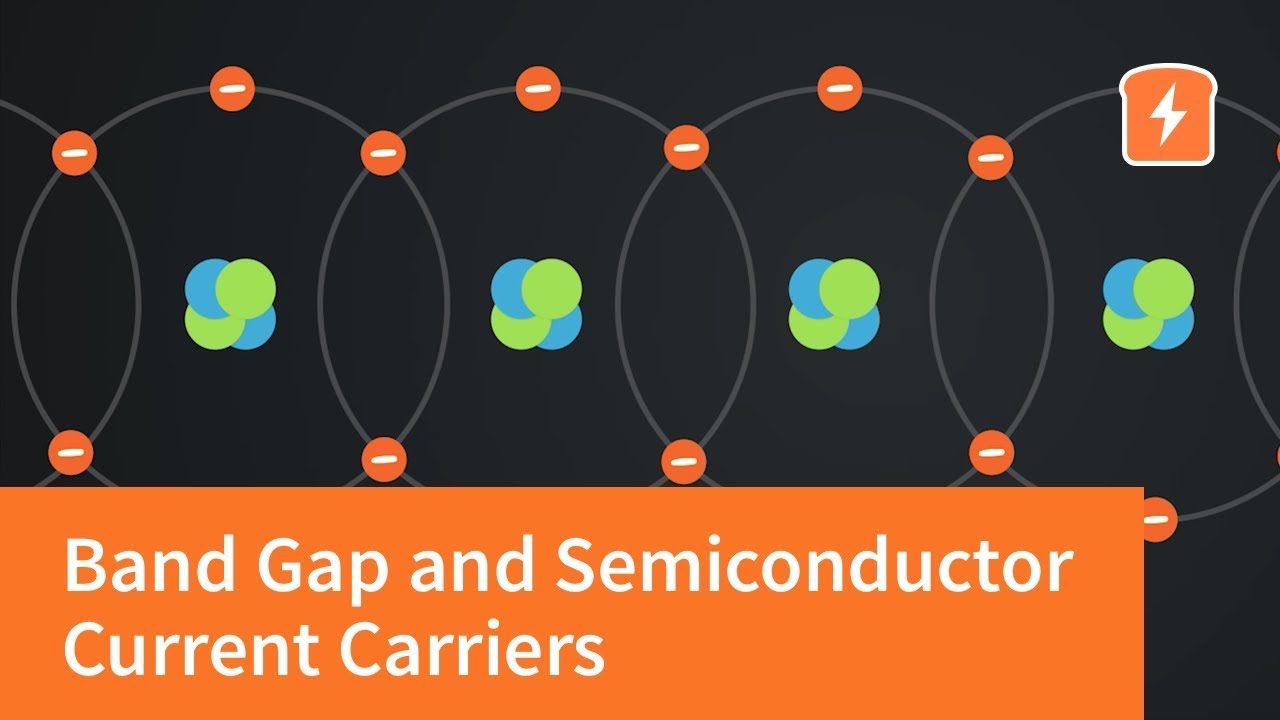
- Electrons Orbiting Atoms: Electrons don't simply exist anywhere around an atom's nucleus; they occupy specific energy levels or orbitals.
- Energy Levels in Solids: When atoms come together to form a solid, their atomic orbitals interact and split into a large number of closely spaced energy levels, forming energy bands.
- Energy Bands and Electrical Conductivity: The arrangement and occupancy of these energy bands determine a material's electrical conductivity.
Delving into the Valence Band
The valence band is crucial for understanding electrical conductivity.
Definition of Valence Band
The valence band represents the range of electron energies where the valence electrons reside. Valence electrons are the outermost electrons of an atom, responsible for chemical bonding. In a solid, the valence band is formed by the overlapping of these valence electron orbitals.
Key Characteristics of the Valence Band
- Highest Occupied Band: At absolute zero (0 Kelvin), the valence band is the highest energy band that is completely filled with electrons.
- Involved in Bonding: The electrons in the valence band are those that participate in the chemical bonds holding the solid together.
- Not Usually Involved in Conduction (on its own): When the valence band is full, electrons can't easily move within the band to conduct electricity because there are no empty states available for them to transition into.
Exploring the Conduction Band
The conduction band holds the key to electrical conductivity.
Definition of Conduction Band
The conduction band is the range of electron energies above the valence band. If electrons are present in the conduction band, they are relatively free to move throughout the material.
Key Characteristics of the Conduction Band
- Higher Energy Band: The conduction band is at a higher energy level than the valence band.
- Normally Empty (in Insulators and Semiconductors at low temperature): Ideally, at absolute zero, the conduction band is empty in insulators and semiconductors.
- Electrons Enable Conduction: Electrons in the conduction band can move freely, allowing the material to conduct electricity.
- Promotion Required: For an electron to move from the valence band to the conduction band, it needs to gain energy. This energy could come from heat, light, or an electric field.
The Energy Gap: Separating the Bands
The energy gap, also known as the band gap, is a critical parameter that defines a material's electrical behavior.
Definition of Band Gap
The band gap is the energy difference between the top of the valence band and the bottom of the conduction band. No allowed electron energy levels exist within this gap.
The Role of the Band Gap
The size of the band gap determines whether a material is an insulator, a semiconductor, or a conductor.
- Insulators: Have a large band gap (typically > 3 eV). Electrons cannot easily jump from the valence band to the conduction band.
- Semiconductors: Have a moderate band gap (typically ~ 1 eV). Electrons can jump to the conduction band with the addition of sufficient energy (e.g., heat, light).
- Conductors: Have either a very small band gap or overlapping valence and conduction bands. Electrons are already readily available for conduction.
Semiconductor Behavior Explained
The magic of semiconductors lies in their ability to control the flow of electrons, which stems from the band gap between the valence and conduction bands.

Excitation of Electrons
Electrons in the valence band can be excited (e.g., by heat or light) and jump the band gap to the conduction band. This creates:
- Free Electrons: Electrons in the conduction band that can move freely and conduct electricity.
- Holes: Vacancies left behind in the valence band when electrons move to the conduction band. Holes behave as positive charge carriers and contribute to conduction as well.
Temperature Dependence
Semiconductor conductivity is highly dependent on temperature. As temperature increases, more electrons have sufficient energy to jump the band gap, increasing conductivity.
Doping: Tailoring Conductivity
The most effective method of controlling semiconductor conductivity is through doping. Doping involves introducing impurities into the semiconductor crystal lattice.
- N-type Doping: Adding impurities with more valence electrons (e.g., phosphorus in silicon) creates extra electrons in the conduction band, increasing electron concentration. These impurities are called donors.
- P-type Doping: Adding impurities with fewer valence electrons (e.g., boron in silicon) creates holes in the valence band, increasing hole concentration. These impurities are called acceptors.
Visualizing Energy Bands: A Table Summary
| Feature | Valence Band | Conduction Band |
|---|---|---|
| Energy Level | Lower Energy | Higher Energy |
| Electron Occupation | Typically Filled (at absolute zero) | Typically Empty (at absolute zero, insulators/semiconductors) |
| Electrons | Bound to Atoms (Involved in bonding) | Free to Move (Conduction) |
| Role in Conduction | Provides electrons (when excited) and holes | Carries electric current |
| Importance | Critical for understanding material properties | Critical for understanding material properties |
Video: Unlock Semiconductor Secrets: Valence vs. Conduction Bands!
Understanding Semiconductor Bands: Your FAQs
Still wrapping your head around valence and conduction bands? Here are some frequently asked questions to help solidify your understanding of semiconductor behavior.
What exactly are the valence and conduction bands in a semiconductor?
The valence band is the highest range of electron energies where electrons are normally present at absolute zero temperature. The conduction band is the lowest range of vacant electron energies. Essentially, the what is valence band and conduction band in semiconductor define the energy levels available for electrons, and their ability to conduct electricity.
How do electrons move between the valence and conduction bands?
Electrons require energy to jump from the valence band to the conduction band. This energy can be supplied in the form of heat (thermal energy), light (photons), or an electric field. When an electron gains enough energy, it can overcome the energy gap and become a free carrier, enabling electrical conductivity.
What is the energy gap, and why is it important?
The energy gap (or band gap) is the energy difference between the top of the valence band and the bottom of the conduction band. It is a key factor determining the electrical conductivity of a semiconductor. A smaller energy gap means it's easier for electrons to move to the conduction band.
What happens when an electron moves from the conduction band back to the valence band?
When an electron falls back from the conduction band to the valence band, it releases energy. This energy can be emitted as a photon (light), contributing to phenomena like light-emitting diodes (LEDs). Understanding what is valence band and conduction band in semiconductor behavior is critical in LED design.