Decoding Work: What Units Measure the Effort? Find Out!

Work, a fundamental concept in physics, finds practical applications across diverse fields like engineering and even everyday tasks. James Prescott Joule, a renowned physicist, significantly contributed to our understanding of this phenomenon. A crucial aspect in understanding work involves knowing what units are used for work done, particularly the Joule (J) itself, which represents the energy transferred when a force of one Newton moves an object one meter in the direction of the force, often analyzed within the framework of classical mechanics.
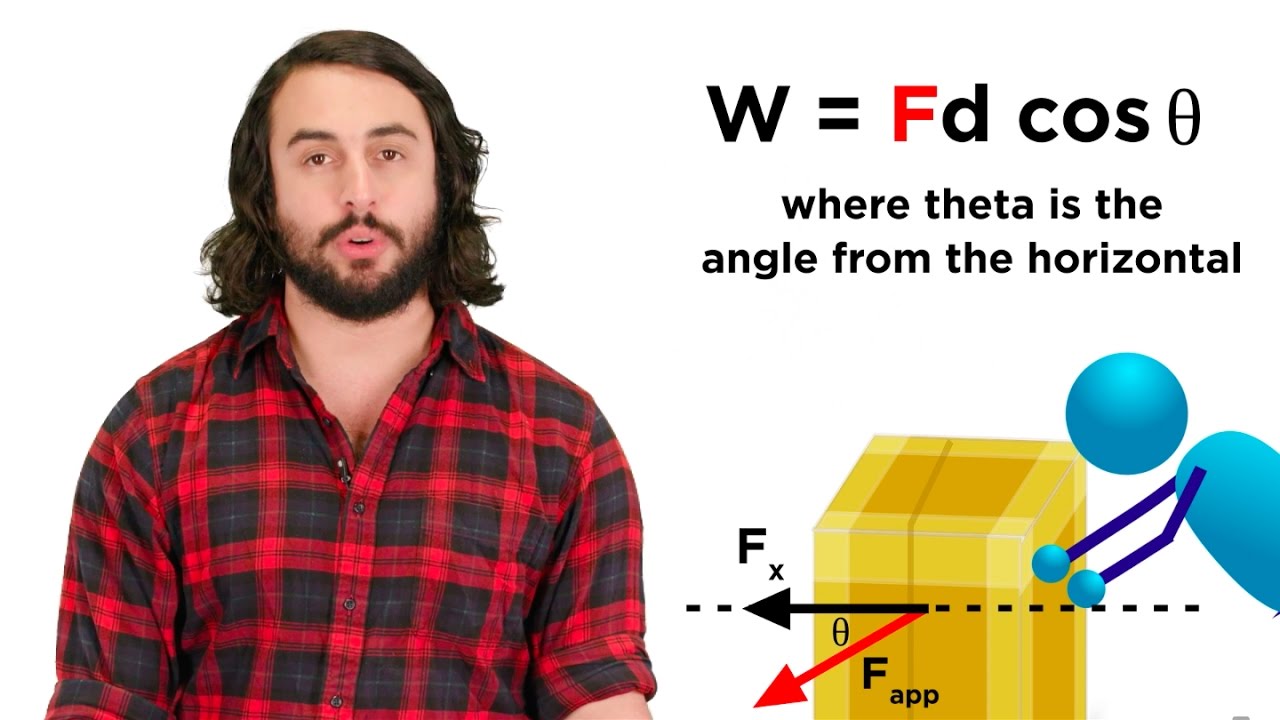
Image taken from the YouTube channel Professor Dave Explains , from the video titled Work and Energy .
Work, in the realm of physics, transcends the everyday notion of effort. It represents a precise quantification of energy transfer, a measure of how force acting upon an object causes its displacement.

From the simple act of lifting a book to the complex workings of an engine, work is the common thread linking force and motion.
But how do we measure something as fundamental as work? This is where the concept of units becomes crucial. We don't just say "work was done"; we quantify how much work was done.
Why Understanding Units of Work is Essential
Imagine trying to describe the length of a table without using inches, centimeters, or any standard unit. The description would be vague and meaningless. Similarly, understanding the units used to measure work is essential for accurate communication and analysis in physics, engineering, and related fields.
The appropriate unit of measurement brings clarity, precision, and allows for meaningful comparisons.
Without a clear understanding of these units, it becomes impossible to compare the efficiency of different machines, calculate the energy requirements of a process, or even understand the fundamental laws of physics.
Thesis: A Journey Through the Landscape of Work Units
This exploration delves into the diverse units used to quantify work, from the familiar Joule to the less common Erg and Foot-Pound. We aim to unravel their definitions, understand their relationships, and appreciate their significance in various contexts.
Join us as we navigate the landscape of work units, equipping ourselves with the tools to accurately measure and understand the energy that shapes our world.
Through this examination, we’ll be able to appreciate the ingenuity behind these measurements and their importance in our ongoing understanding of the physical world.
Work, in the realm of physics, transcends the everyday notion of effort. It represents a precise quantification of energy transfer, a measure of how force acting upon an object causes its displacement.

From the simple act of lifting a book to the complex workings of an engine, work is the common thread linking force and motion.
But how do we measure something as fundamental as work? This is where the concept of units becomes crucial. We don't just say "work was done"; we quantify how much work was done.
Why Understanding Units of Work is Essential
Imagine trying to describe the length of a table without using inches, centimeters, or any standard unit. The description would be vague and meaningless. Similarly, understanding the units used to measure work is essential for accurate communication and analysis in physics, engineering, and related fields.
The appropriate unit of measurement brings clarity, precision, and allows for meaningful comparisons.
Without a clear understanding of these units, it becomes impossible to compare the efficiency of different machines, calculate the energy requirements of a process, or even understand the fundamental laws of physics.
Thesis: A Journey Through the Landscape of Work Units
This exploration delves into the diverse units used to quantify work, from the familiar Joule to the less common Erg and Foot-Pound. We aim to unravel their definitions, understand their relationships, and appreciate their significance in various contexts.
Join us as we navigate the landscape of work units, equipping ourselves with the tools to accurately…
Defining Work: Force, Distance, and Energy
Having established the significance of understanding work units, it's paramount to define work itself, as understood within the context of physics. This requires moving beyond the everyday, subjective understanding of "work" and embracing a precise, quantifiable definition.
Work as Force Applied Over a Distance
In physics, work is defined as the energy transferred to or from an object by a force acting on the object causing a displacement. Crucially, work is done only if the object moves while the force is applied.
Simply applying a force is not enough. If you push against a stationary wall, you exert a force, but since the wall doesn't move, no work is done in the physics sense.
The Interplay of Force and Displacement
The relationship between force and displacement is fundamental to understanding work. Work is done when a force causes an object to move a certain distance in the direction of the force.
If the force and displacement are in the same direction, the work done is positive. This signifies that energy is being transferred to the object, increasing its kinetic or potential energy.
However, if the force and displacement are in opposite directions, the work done is negative. This means the object is losing energy, perhaps due to friction or a force opposing its motion.
Consider pushing a box across the floor. The force you apply and the displacement of the box are in the same direction, resulting in positive work.
Now, imagine the box sliding to a stop due to friction. The frictional force acts opposite to the box's motion, doing negative work and reducing its kinetic energy.
The Formula for Calculating Work
The mathematical representation of work is elegantly simple:
W = F ⋅ d ⋅ cos(θ)
Where:
- W represents work,
- F represents the magnitude of the force,
- d represents the magnitude of the displacement, and
- θ represents the angle between the force and displacement vectors.
If the force and displacement are in the same direction (θ = 0°), then cos(θ) = 1, and the equation simplifies to:
W = F ⋅ d
This simplified version is applicable when the force is directly aligned with the direction of motion.
The Intimate Relationship Between Energy and Work
Work and energy are inextricably linked. Work is, in essence, the transfer of energy. When work is done on an object, its energy changes. This change can manifest as a change in kinetic energy (energy of motion), potential energy (energy of position), or both.
The Work-Energy Theorem formalizes this relationship: it states that the net work done on an object is equal to the change in its kinetic energy.
Wnet = ΔKE = KEfinal - KE_initial
This theorem provides a powerful tool for analyzing motion. If we know the net work done on an object, we can determine its change in kinetic energy and, consequently, its change in speed. Conversely, if we know the change in kinetic energy, we can determine the net work done.
Understanding this connection between work and energy is critical for comprehending a wide range of physical phenomena, from the motion of projectiles to the operation of machines.
Work, as we've seen, is more than just a feeling of exertion; it’s a precisely defined quantity. To give that quantity meaning, we need a standardized way to measure it.
The Joule: The Standard Unit Explained
The Joule (symbol: J) stands as the cornerstone unit for measuring energy and, consequently, work within the International System of Units (SI). It's the unit you'll encounter most frequently in scientific literature, engineering calculations, and everyday applications involving energy. Understanding the Joule is crucial for anyone seeking to grasp the quantitative aspects of work and energy.
Defining the Joule in Fundamental Terms
What exactly is a Joule? At its core, a Joule represents the amount of work done when a force of one Newton (N) displaces an object by a distance of one meter (m) in the direction of the force. This definition directly stems from the formula for work:
Work (W) = Force (F) × Distance (d)
Therefore, 1 Joule (J) = 1 Newton (N) × 1 meter (m).
Breaking it down further, a Newton is defined as the force required to accelerate a mass of one kilogram (kg) at a rate of one meter per second squared (m/s²). This leads to a more fundamental expression of the Joule:
1 J = 1 kg ⋅ m²/s²
This reveals the Joule as a composite unit, built upon the base SI units of kilogram, meter, and second. This interconnectedness highlights the elegance and consistency of the SI system.
James Prescott Joule: The Man Behind the Unit
The Joule isn't just a collection of symbols; it's named in honor of James Prescott Joule (1818-1889), a British physicist whose groundbreaking experiments established the relationship between heat and mechanical work.
Joule's meticulous measurements demonstrated that mechanical work could be converted into heat, and vice versa, with a fixed proportionality. This led to the formulation of the law of conservation of energy, a cornerstone of thermodynamics. Naming the SI unit of energy after Joule recognizes his pivotal role in understanding the fundamental connection between work, energy, and heat.
The Work-Energy Theorem and the Significance of Joules
The Work-Energy Theorem provides a powerful link between work and kinetic energy. It states that the net work done on an object is equal to the change in its kinetic energy. Mathematically, this is expressed as:
W = ΔKE = KEfinal - KEinitial
Where:
- W is the work done
- ΔKE is the change in kinetic energy
- KEfinal is the final kinetic energy
- KEinitial is the initial kinetic energy
This theorem is particularly useful when working with Joules, as it provides a direct connection between the work done on an object (measured in Joules) and its change in motion (also expressible in terms of Joules through kinetic energy).
For example, if we know that 10 Joules of work are done on an object, we can directly infer that its kinetic energy has increased by 10 Joules. The Work-Energy Theorem, expressed using Joules as the unit of measurement, offers a clear and quantitative understanding of how work affects the motion of objects.
Exploring Alternative Units: Erg and Foot-Pound
While the Joule reigns supreme in the SI system, the world of work and energy isn't exclusively defined by it. Two other units, the Erg and the Foot-Pound, have historical significance and, in some niche applications, continue to be used.
Understanding these alternative units broadens our perspective and helps us appreciate the evolution of scientific measurement.
The Erg: A CGS Unit
The Erg (symbol: erg) is a unit of energy and work in the Centimetre-Gram-Second (CGS) system of units. It's a significantly smaller unit compared to the Joule, reflecting the smaller base units used in the CGS system.
Definition and Conversion
One Erg is defined as the amount of work done when a force of one dyne displaces an object by one centimetre.
Mathematically, 1 erg = 1 dyne ⋅ cm.
Since 1 dyne is equal to 1 gram ⋅ cm/s², we can express the Erg in terms of CGS base units as:
1 erg = 1 g ⋅ cm²/s².
To convert Ergs to Joules, we use the following relationship:
1 Joule = 107 Ergs, or conversely, 1 Erg = 10-7 Joules.
This conversion factor highlights the substantial difference in scale between the two units.
Historical Context and Use Cases
The Erg was widely used in scientific research, particularly in fields like spectroscopy and atomic physics, before the widespread adoption of the SI system. Its smaller scale made it convenient for expressing the tiny amounts of energy involved in these phenomena.
While largely superseded by the Joule in modern scientific practice, the Erg may still be encountered in older literature or in specialized contexts where CGS units persist.
The Foot-Pound: An Imperial Unit
The Foot-Pound (symbol: ft⋅lb or ft⋅lbf) is a unit of work or energy in the Imperial and United States customary systems of units. It represents the amount of work required to raise a one-pound weight a distance of one foot against gravity.
Definition and Conversion
Specifically, the foot-pound is defined as the work done by a force of one pound-force acting through a distance of one foot in the direction of the force.
Converting Foot-Pounds to Joules requires using the following approximate relationship:
1 Foot-Pound ≈ 1.356 Joules.
Conversely, 1 Joule ≈ 0.738 Foot-Pounds.
Relevance and Applications
The Foot-Pound is commonly encountered in engineering, particularly in the United States, where the Imperial system still sees use. It's frequently used to express torque, a rotational force closely related to work.
For example, the torque output of an engine might be specified in foot-pounds.
While the SI system is increasingly adopted in engineering, the Foot-Pound remains relevant in contexts where familiarity with Imperial units is paramount.
When Alternative Units Are Preferred
The choice of unit often depends on the specific field, the historical context, and the preferences of the individuals or organizations involved.
In some cases, older literature may exclusively use Ergs or Foot-Pounds, necessitating familiarity with these units for proper interpretation.
Certain industries, particularly in the United States, may continue to use Foot-Pounds due to established practices and regulatory requirements.
Ultimately, understanding the relationships between different units of work allows for seamless conversion and interpretation of data across various contexts.
The Erg and Foot-Pound, while not as ubiquitous as the Joule, offer valuable insights into the diverse ways work has been measured. Understanding their definitions and historical applications enriches our appreciation for the evolution of scientific measurement. But quantifying work is only part of the story. To truly understand the dynamics of physical processes, we must consider how quickly that work is performed.
Power: The Rate of Doing Work
Power is a fundamental concept in physics that describes the rate at which work is done or energy is transferred. It tells us not just how much work is accomplished, but how fast that work is completed.
This understanding is critical in countless real-world applications, from designing efficient engines to analyzing the energy consumption of household appliances.
Defining Power
Power is defined as the amount of work done per unit of time. In simpler terms, it's the speed at which work is performed. A high-power device can perform a large amount of work in a short time, while a low-power device will take longer to accomplish the same task.
The Watt: The Unit of Power
The standard unit of power in the International System of Units (SI) is the Watt (symbol: W).
One Watt is defined as one Joule of work done per second: 1 W = 1 J/s.
The Watt is named after James Watt, the Scottish inventor who significantly improved the steam engine, a device that revolutionized industry and transportation.
His contributions were so significant that the unit of power was named in his honor, solidifying his place in the history of science and engineering.
The Relationship Between Power, Work, and Time
The relationship between power (P), work (W), and time (t) can be expressed by the following formula:
P = W / t
This equation highlights that power is directly proportional to work and inversely proportional to time. This means:
-
If you increase the amount of work done in the same amount of time, you increase the power.
-
If you do the same amount of work in a shorter amount of time, you also increase the power.
Understanding this relationship is crucial for analyzing and optimizing the performance of various systems and devices.
Practical Examples
Power calculations are essential in many everyday situations:
Lifting Objects
Imagine lifting a box from the floor to a table. The work done is the force you exert (equal to the box's weight) multiplied by the distance you lift it.
The power you exert depends on how quickly you lift the box. If you lift it quickly, you exert more power than if you lift it slowly.
Pushing a Car
Consider pushing a stalled car. The work done is the force you apply to the car multiplied by the distance you push it.
The power you exert depends on how quickly you can push the car. A stronger person might be able to push the car with more power than a weaker person, meaning they can achieve the same amount of work in less time.
Electrical Appliances
Household appliances are rated in Watts, indicating their power consumption. A higher wattage appliance consumes more energy per unit of time than a lower wattage appliance.
Understanding the power ratings of appliances allows consumers to make informed decisions about energy usage and costs.
The ability to quantify power offers a powerful lens through which to view physical processes. But how does this understanding translate into more complex systems, like those studied in thermodynamics and mechanics? Let's delve into these realms to explore the multifaceted role of work.
Work in Thermodynamics and Mechanical Systems
The concept of work plays a pivotal role in both thermodynamics and mechanical systems, serving as a bridge between energy transfer and observable changes. Understanding how work manifests in these domains is essential for analyzing the efficiency and performance of various engineering applications.
Thermodynamics and Work
Thermodynamics, at its core, deals with the relationships between heat, work, and energy. In thermodynamic systems, work is often associated with changes in volume or pressure. For instance, consider a gas enclosed within a cylinder.
If the gas expands against a piston, it performs work on the surroundings. Conversely, if the gas is compressed, work is done on the gas. This pressure-volume work is fundamental to understanding the operation of engines, refrigerators, and other thermodynamic devices.
The amount of work done in a thermodynamic process depends not only on the initial and final states but also on the path taken. This path-dependency highlights the importance of understanding the specific processes involved.
Measuring Thermodynamic Work
Several key concepts are used to measure the amount of work done in thermodynamics. Enthalpy, for example, is a thermodynamic property that accounts for the internal energy of a system plus the product of its pressure and volume. Changes in enthalpy are particularly useful for analyzing processes that occur at constant pressure, such as many chemical reactions.
Entropy, another crucial concept, is a measure of the disorder or randomness of a system. While not directly a measure of work, changes in entropy are intimately linked to the efficiency of thermodynamic processes. The Second Law of Thermodynamics dictates that the total entropy of an isolated system can only increase over time, implying that not all energy can be converted into useful work.
Heat transfer is another concept used in measuring the amount of work done in thermodynamics. Heat transfer is the exchange of thermal energy between systems and affects the amount of work that can be done.
Mechanical Work
Mechanical work, in its simplest form, involves the application of force over a distance, as discussed earlier. However, in mechanical systems, work often manifests in more complex ways. For example, consider a rotating machine.
The work done by the machine is related to the torque applied and the angular displacement of the rotating parts. Similarly, in systems involving friction, some of the input work is dissipated as heat, reducing the overall efficiency.
Work and Energy Transfer
Work in mechanical systems is inextricably linked to energy transfer. When work is done on an object, its kinetic or potential energy changes.
For example, lifting an object increases its gravitational potential energy, while accelerating an object increases its kinetic energy. Understanding these energy transformations is crucial for designing efficient mechanical systems.
The Usefulness of Work: Applications and Implications
The ability to harness and control work is fundamental to countless technologies that shape our modern world.
From power generation to transportation, and manufacturing processes, work enables us to perform tasks that would otherwise be impossible. Efficiently converting energy into useful work is a central goal of engineering, driving innovation in areas such as engine design, renewable energy technologies, and materials science.
Moreover, a thorough understanding of work allows us to analyze and improve existing systems. By identifying sources of energy loss and optimizing the way work is performed, we can create more sustainable and efficient technologies.
Video: Decoding Work: What Units Measure the Effort? Find Out!
FAQs: Decoding Work - Understanding the Units of Effort
Here are some frequently asked questions about understanding the units used to measure work, helping you decode the effort involved in physical tasks.
What exactly does "work" mean in physics, and how is it different from everyday use?
In physics, "work" specifically refers to the energy transferred when a force causes displacement. This means something must move for work to be done. Everyday usage is broader, encompassing mental effort or simply being employed. Work, in physics, is quantified using what units are used for work done, typically Joules.
What units are used to measure work done, and how are they derived?
The standard unit for measuring work is the Joule (J). One Joule is defined as the amount of work done when a force of one Newton moves an object one meter in the direction of the force. So, 1 J = 1 N⋅m. Therefore, Joules are the key to understanding what units are used for work done.
Can work be negative, and if so, what does that indicate?
Yes, work can be negative. Negative work happens when the force applied acts in the opposite direction to the displacement. This implies that energy is being taken away from the object, like friction slowing something down. Still measured using Joules, it's an important nuance for what units are used for work done.
How does the angle between the force and displacement affect the work done?
The angle matters significantly. Only the component of the force acting in the direction of the displacement contributes to the work. If the force is perpendicular to the displacement, no work is done (even if there's force and movement!). The cosine of the angle is used in the work calculation: Work = Force x Distance x cos(angle), influencing what units are used for work done calculation.
