SN2 Unreactive Alkyl Halides: The Shocking Truth!

The SN2 reaction mechanism, a fundamental concept in organic chemistry, is profoundly influenced by the structure of the alkyl halide. Consequently, steric hindrance, a key factor elucidated by Professor Christopher Ingold's pioneering work, dramatically impacts reactivity. Our investigation reveals the surprising truth about tertiary alkyl halides, demonstrating that their bulky structure makes it unlikely that which of the alkyl halides shown is essentially unreactive in an sn2 reaction. This inherent unreactivity stems from the backside attack being significantly obstructed, impacting reaction rates analyzed by numerous computational chemistry labs.

Image taken from the YouTube channel kwokthechemteacher , from the video titled Alkyl Halides (SN2).mp4 .
Why do some molecules stubbornly resist reactions that others readily embrace?
In the realm of organic chemistry, few transformations are as fundamental and widely utilized as the SN2 reaction.
This seemingly simple process, where a nucleophile attacks a carbon atom, displacing a leaving group, underpins a vast array of synthetic methodologies.
Yet, the efficiency of SN2 reactions is far from uniform.
Some alkyl halides, the substrates upon which SN2 reactions act, exhibit an almost defiant inertness, confounding expectations and demanding a deeper understanding.
This article aims to unravel the mystery behind these exceptionally sluggish reactants.
Our focus is to pinpoint and elucidate the structural features that render specific alkyl halides, particularly tertiary and neopentyl halides, essentially unreactive in SN2 reactions.
This investigation is directly relevant to the practical challenge of predicting and explaining the reactivity of different alkyl halides in SN2 reactions.
Specifically, addressing the query: "which of the alkyl halides shown is essentially unreactive in an SN2 reaction?" requires a nuanced understanding of steric and electronic factors.
SN2 Reactions: A Brief Overview
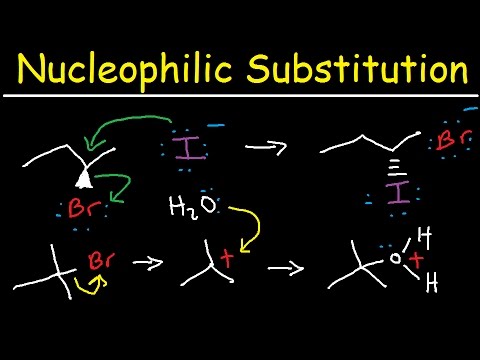
The SN2 reaction, short for bimolecular nucleophilic substitution, is a cornerstone of organic synthesis.

It involves a single-step mechanism where a nucleophile attacks the substrate carbon from the backside, simultaneously displacing the leaving group.
This concerted process results in an inversion of stereochemistry at the reaction center, often referred to as the Walden inversion.
The reaction's rate is dependent on the concentration of both the nucleophile and the substrate, hence the term "bimolecular."
Why SN2 Reactions Matter
SN2 reactions are crucial because they provide a direct and predictable method for introducing new functional groups into organic molecules.
Their utility extends across diverse fields, from drug discovery and materials science to polymer chemistry and agrochemicals.
However, the effectiveness of SN2 reactions hinges on several factors, including the structure of the alkyl halide substrate.
Certain structural features can dramatically impede or even prevent the SN2 reaction from occurring, despite favorable conditions.
The Quest for Unreactive Alkyl Halides
Our primary objective is to identify and explain why specific alkyl halides are virtually inert in SN2 reactions.
Specifically, we are interested in alkyl halides that present significant steric hindrance to the incoming nucleophile, effectively blocking the backside attack required for the SN2 mechanism to proceed.
Understanding which alkyl halides are virtually inert in SN2 reactions is not merely an academic exercise.
It has practical implications in reaction design, allowing chemists to strategically select reactants and conditions to achieve desired transformations with optimal efficiency and selectivity.
By delving into the structural characteristics of these unreactive alkyl halides, we will gain valuable insights into the factors governing SN2 reactivity and unlock strategies for overcoming these limitations.
Why some molecules stubbornly resist reactions that others readily embrace? In the realm of organic chemistry, few transformations are as fundamental and widely utilized as the SN2 reaction. This seemingly simple process, where a nucleophile attacks a carbon atom, displacing a leaving group, underpins a vast array of synthetic methodologies. Yet, the efficiency of SN2 reactions is far from uniform. Some alkyl halides, the substrates upon which SN2 reactions act, exhibit an almost defiant inertness, confounding expectations and demanding a deeper understanding. This article aims to unravel the mystery behind these exceptionally sluggish reactants. Our focus is to pinpoint and elucidate the structural features that render specific alkyl halides, particularly tertiary and neopentyl halides, essentially unreactive in SN2 reactions. This investigation is directly relevant to the practical challenge of predicting and explaining the reactivity of different alkyl halides in SN2 reactions. Specifically, addressing the query: "which of the alkyl halides shown is essentially unreactive in an SN2 reaction?" requires a nuanced understanding of steric and electronic factors. SN2 Reactions: A Brief Overview The SN2 reaction, short for bimolecular nucleophilic substitution, is a cornerstone of organic synthesis. It involves a single-step mechanism where a nucleophile attacks the substrate carbon from the backside, simultaneously displacing the leaving group. This concerted process results in an inversion of stereochemistry at the reaction center, often referred to as the Walden inversion. The reaction's rate is dependent on the concentration of both the nucleophile and the substrate, hence the term "bimolecular." Why SN2 Reactions Matter...
Understanding the nuances of the SN2 reaction requires more than just knowing its definition. It's crucial to delve into the mechanics, the actors involved, and the choreography of their interactions. This foundational knowledge sets the stage for understanding why certain alkyl halides are so resistant to undergoing this seemingly straightforward transformation.
SN2 Reaction Mechanism: A Deep Dive
At its core, the SN2 reaction embodies a meticulously orchestrated molecular dance. It's a single-step, concerted process where bond formation and bond breakage occur simultaneously. This characteristic is fundamentally different from reactions that proceed through discrete, multi-step intermediates. Understanding this concerted nature is key to appreciating the factors that govern SN2 reactivity.
The Concerted Mechanism Unveiled
Imagine a nucleophile, an electron-rich species, approaching a substrate carbon bearing a leaving group. The nucleophile attacks from the backside, precisely 180 degrees opposite the leaving group. This backside attack is a hallmark of the SN2 mechanism and dictates the stereochemical outcome.
As the nucleophile approaches, it begins to form a bond with the carbon atom, while simultaneously the bond between the carbon and the leaving group weakens. This leads to the formation of a transition state, a high-energy, pentavalent species where the carbon is partially bonded to both the nucleophile and the leaving group.
The transition state is fleeting, representing the peak of the energy barrier that the reaction must overcome. Once the transition state is reached, the leaving group departs, taking its bonding electrons with it. The nucleophile is now fully bonded to the carbon, and the stereochemistry at the carbon center is inverted, much like an umbrella turning inside out in a strong wind—the Walden inversion.
The Actors: Nucleophile and Leaving Group
The SN2 reaction hinges on the properties of two crucial players: the nucleophile and the leaving group.
The Nucleophile: The Attacking Agent
The nucleophile, meaning "nucleus-loving," is an electron-rich species capable of donating a pair of electrons to form a new bond. The strength of the nucleophile significantly impacts the reaction rate. Stronger nucleophiles, those with a greater tendency to donate electrons, drive the reaction forward more effectively.
Factors that influence nucleophilicity include charge, electronegativity, and steric hindrance within the nucleophile itself. For example, a negatively charged nucleophile is generally stronger than its neutral counterpart.
The Leaving Group: Making a Graceful Exit
The leaving group is an atom or group of atoms that departs from the substrate, taking its bonding electrons with it. A good leaving group must be able to stabilize the negative charge it acquires upon departure. Generally, weak bases make good leaving groups because they are stable as anions.
Common examples include halide ions (I-, Br-, Cl-), and tosylate (TsO-) groups. The nature of the leaving group significantly affects the SN2 reaction rate; better leaving groups lead to faster reactions. The leaving group's ability to stabilize the negative charge it carries away directly influences the ease with which the SN2 reaction proceeds.
Factors Influencing SN2 Reaction Rates
Several factors dictate the speed at which an SN2 reaction occurs. Understanding these factors is paramount in predicting and controlling reaction outcomes.
Substrate Structure: The Accessibility Factor
The structure of the alkyl halide substrate plays a pivotal role. Steric hindrance, the crowding of atoms or groups around the reaction center, is a major impediment. Methyl and primary alkyl halides are highly reactive in SN2 reactions because the nucleophile has easy access to the carbon atom.
As the degree of substitution on the carbon increases (secondary, tertiary), the reaction rate slows dramatically due to the increased steric bulk obstructing the nucleophile's approach.
Nucleophile Strength: The Driving Force
As mentioned earlier, the strength of the nucleophile directly correlates with the reaction rate. Stronger nucleophiles attack more readily, accelerating the reaction.
Solvent Effects: Polar Aprotic Favors SN2
The solvent in which the reaction is carried out can also have a significant impact. Polar aprotic solvents, such as acetone, DMSO, and DMF, are particularly effective for SN2 reactions. These solvents dissolve polar reactants but do not solvate nucleophiles as strongly as protic solvents (like water or alcohols), leaving them more available to attack the substrate.
By understanding the interplay of these factors – the concerted mechanism, the roles of the nucleophile and leaving group, and the influence of substrate structure and solvent – we gain a deeper appreciation for the SN2 reaction and the reasons behind its variable efficiency. This foundation is critical for the discussion of steric hindrance and the unreactivity of certain alkyl halides that will follow.
SN2 reactions are nuanced dances between nucleophiles and electrophiles, where the stage itself—the molecular structure—plays a pivotal role.
The rate at which these reactions proceed isn’t solely dictated by the inherent reactivity of the participating molecules.
Rather, it's often determined by the accessibility of the reaction site.
Steric Hindrance: The Major Obstacle to SN2 Reactions
At the heart of many sluggish or completely inhibited SN2 reactions lies the concept of steric hindrance.
This phenomenon arises from the spatial bulk of substituents surrounding the reactive carbon atom.
It's a critical factor governing the feasibility and rate of SN2 reactions.
Understanding Steric Hindrance
Steric hindrance, in its essence, refers to the obstruction of a chemical reaction due to the size and arrangement of atoms or groups of atoms within a molecule.
In the context of SN2 reactions, this obstruction manifests as a physical barrier.
Bulky groups surrounding the carbon atom undergoing nucleophilic attack impede the approach of the nucleophile.
Imagine trying to thread a needle in a crowded room; the surrounding people (bulky groups) make the task significantly more difficult.
The Backside Attack and Steric Clutter
The SN2 mechanism mandates a backside attack of the nucleophile, an approach that is directly opposite the leaving group.
This requirement makes the reaction exceptionally sensitive to steric crowding.
As the size of the substituents attached to the carbon increases, the space available for the nucleophile to approach diminishes.
This congestion makes it harder for the nucleophile to effectively interact with the electrophilic carbon center.
Steric Hindrance and Alkyl Halide Structure
The structure of the alkyl halide is directly related to the degree of steric hindrance it presents.
Primary alkyl halides, with only one alkyl group attached to the reactive carbon, offer the least steric hindrance.
This makes them the most reactive in SN2 reactions.
Secondary alkyl halides, bearing two alkyl groups, exhibit more hindrance, leading to slower reaction rates.
Tertiary alkyl halides, with three alkyl groups surrounding the carbon, are the most sterically encumbered.
The bulky groups effectively shield the carbon from nucleophilic attack.
This shielding renders them virtually unreactive under normal SN2 conditions.
Furthermore, neopentyl halides, despite being primary, exhibit significant steric hindrance due to the presence of a bulky tert-butyl group attached to the adjacent carbon.
This arrangement creates substantial crowding around the reaction center.
This prevents the nucleophile from effectively reaching the electrophilic carbon.
In essence, steric hindrance is not merely a passive impediment, but an active barrier that can effectively shut down an SN2 reaction.
The shape and size of the alkyl halide dictates the degree to which this obstacle influences the reaction's outcome.
Steric hindrance, however, is not a uniform barrier; its impact varies significantly depending on the specific alkyl halide in question. While all SN2 reactions are susceptible to this phenomenon to some degree, certain structural motifs amplify the steric demands to the point of rendering the reaction virtually impossible.
The Prime Suspects: Tertiary and Neopentyl Halides
Among the alkyl halides, tertiary and neopentyl halides stand out as particularly unreactive substrates in SN2 reactions. Their unique structures create an environment of extreme steric congestion around the reactive carbon center, effectively shutting down the possibility of a successful backside attack.
Tertiary Alkyl Halides: A Crowded Carbon
Tertiary alkyl halides are characterized by a carbon atom bonded to the halogen and three other carbon-containing groups. This arrangement inherently creates a substantial degree of steric hindrance.
The three alkyl groups, regardless of their size, occupy significant space around the central carbon.
This spatial bulk makes it exceedingly difficult for a nucleophile to approach the carbon from the backside, which is a fundamental requirement of the SN2 mechanism.
The transition state, where the nucleophile is partially bonded to the carbon while the leaving group departs, is particularly sensitive to steric crowding.
In tertiary alkyl halides, the transition state becomes so energetically unfavorable due to steric clashes that the SN2 reaction is effectively shut down.
Neopentyl Halides: The Ultimate Steric Challenge
Neopentyl halides represent an even more extreme case of steric hindrance.
A neopentyl halide features a halogen bonded to a carbon atom that is directly attached to a tertiary butyl group (a carbon bonded to three methyl groups).
This seemingly small structural modification has a profound impact on SN2 reactivity.
The bulky tertiary butyl group effectively shields the reactive carbon center, creating an insurmountable barrier for the approaching nucleophile.
Imagine trying to access a door that is blocked by a massive pile of furniture; this is analogous to the steric environment surrounding the reactive carbon in a neopentyl halide.
The Impact of Remote Steric Hindrance
What makes neopentyl halides particularly interesting is that the steric hindrance is not directly attached to the reacting carbon itself.
It is located one carbon away.
This "remote" steric hindrance is still potent enough to completely inhibit SN2 reactions.
The methyl groups of the tertiary butyl group rotate and block the approach of the nucleophile.
The congestion is similar to a crowded airport security line, preventing anything from getting through quickly or easily.
Unreactive Under Standard SN2 Conditions
Due to the extreme steric hindrance, tertiary and neopentyl halides are considered virtually unreactive under typical SN2 conditions.
While it might be theoretically possible to force an SN2 reaction to occur under extremely harsh conditions (e.g., using a very strong nucleophile at high temperatures), such conditions are generally impractical and can lead to other unwanted side reactions, such as elimination.
In most practical scenarios, chemists avoid attempting SN2 reactions with tertiary and neopentyl halides, opting instead for alternative reaction pathways that are more suitable for these sterically hindered substrates.
The Supporting Cast: The Role of the Leaving Group
While steric hindrance often steals the spotlight when discussing SN2 unreactivity, it's crucial to remember that a successful SN2 reaction hinges on more than just an accessible reaction site. The leaving group, often an overlooked player, is absolutely essential for the reaction to proceed at all. Without a competent leaving group, even the most unhindered alkyl halide will remain stubbornly inert.
The Leaving Group's Essential Function
The SN2 mechanism is, by definition, a simultaneous process. The nucleophile attacks the carbon center from one side, while the leaving group departs from the opposite side, all in a single concerted step. The breaking of the carbon-leaving group bond is inextricably linked to the formation of the carbon-nucleophile bond.
Therefore, the ability of the leaving group to readily detach, taking with it the electron pair from the broken bond, is paramount. The more easily the leaving group departs, the faster the SN2 reaction will proceed.
Defining a "Good" Leaving Group
So, what constitutes a "good" leaving group? Several key properties dictate a leaving group's ability to facilitate an SN2 reaction. The most important factor is the stability of the leaving group as an anion after it departs from the molecule.
Anion Stability: The Key Criterion
A stable anion is one that can effectively accommodate the negative charge. Several factors contribute to anionic stability:
-
Electronegativity: More electronegative atoms are better at stabilizing negative charges. Halogens (iodide, bromide, chloride) are classic examples of good leaving groups due to their electronegativity.
-
Size: Larger atoms can better disperse the negative charge over a larger volume, increasing stability. This is why iodide (I-) is a better leaving group than fluoride (F-), despite fluorine being more electronegative.
-
Resonance: If the leaving group can delocalize the negative charge through resonance, it becomes much more stable. This is why certain sulfonates, such as tosylate (OTs), are excellent leaving groups.
The Inverse Relationship with Basicity
It's important to understand the relationship between a leaving group's ability to depart and its basicity. Good leaving groups are weak bases. Strong bases, which readily donate electrons, are less likely to leave with a pair of electrons.
Think of it this way: a strong base is "happy" holding onto its electrons and is therefore unwilling to depart. Conversely, a weak base is less attached to its electrons and is more willing to leave, taking those electrons with it.
Leaving Group Impact on SN2 Reaction Rate
The nature of the leaving group has a direct impact on the SN2 reaction rate. A better leaving group will result in a faster reaction. This can be experimentally observed by comparing the rates of SN2 reactions with different leaving groups, all other factors being equal.
The relative leaving group ability is often expressed in terms of reaction rates, with common leaving groups ordered as follows (from best to worst):
I- > Br- > Cl- > F- > H2O > HO- > RO- > NH2- > R-
This series emphasizes the critical role of anionic stability and the inverse relationship with basicity in determining leaving group competence. In fact, some of the worst leaving groups, such as HO-, RO-, NH2- and R- are so poor that they almost never leave under SN2 reaction conditions.
The previous sections have highlighted the crucial role of the leaving group in SN2 reactions and the stability of the anion it forms upon departure. But even with a stellar leaving group in place, the reaction can grind to a halt if the approach to the reactive carbon is sufficiently blocked. This brings us to the pivotal concept of steric hindrance and its impact on SN2 reactivity.
Visualizing Steric Hindrance: Examples and Comparisons
Steric hindrance, at its core, is a straightforward concept: the physical blockage of a reaction site by bulky groups. However, understanding its profound effect on SN2 reactions requires a visual grasp of how different alkyl halide structures interact with the incoming nucleophile.
The Visual Impact of Alkyl Halide Structure
Imagine the SN2 reaction as a coordinated dance. The nucleophile must approach the carbon atom from the backside, precisely 180 degrees opposite the leaving group. Any bulky substituents clustered around that carbon atom act as clumsy dance partners, bumping into the nucleophile and disrupting the choreography.
Diagrams illustrating these interactions are essential. For instance, a methyl halide (CH3X) presents minimal steric hindrance. The three hydrogen atoms are relatively small and offer little resistance to the nucleophile's approach.
A primary alkyl halide (RCH2X) experiences slightly more hindrance due to the presence of a larger alkyl group (R). A secondary alkyl halide (R2CHX) faces even greater opposition, as two alkyl groups crowd the reaction center.
It's the tertiary alkyl halide (R3CX) that represents the steric dead end for SN2 reactions. Three alkyl groups completely shield the carbon atom, making backside attack virtually impossible.
Comparing Reactivity: A Visual Spectrum
The difference in reactivity between these halides is dramatic and visually compelling. We can represent this with a spectrum, ranging from highly reactive to essentially unreactive:
Methyl > Primary > Secondary >> Tertiary
This spectrum isn't merely theoretical. It reflects real-world observations. Methyl and primary halides readily undergo SN2 reactions, secondary halides react more slowly, and tertiary halides are, for all practical purposes, inert under typical SN2 conditions.
Neopentyl Halides: The Exception That Proves the Rule
Neopentyl halides (CH3)3CCH2X, present a fascinating case study in steric hindrance. While technically primary halides (the halogen is attached to a primary carbon), they exhibit extremely low SN2 reactivity, often comparable to tertiary halides.
This is because the three methyl groups attached to the beta-carbon create a significant steric bulk that blocks the nucleophile's approach. A visual representation of the neopentyl halide structure clearly illustrates this point.
The Transition State: Where Steric Hindrance Takes Center Stage
The transition state of an SN2 reaction is a pentavalent species where the carbon atom is partially bonded to both the nucleophile and the leaving group. This is the highest energy point in the reaction, and it's here that steric hindrance exerts its maximum influence.
Bulky groups not only impede the initial approach of the nucleophile but also destabilize the transition state itself. The crowding around the carbon atom raises the energy of the transition state, making the reaction slower.
Diagrams of the transition states for primary, secondary, and tertiary halides vividly demonstrate this effect. The more crowded the transition state, the higher the activation energy and the slower the reaction.
By visualizing the steric environment around the reaction center and understanding how it affects both the nucleophile's approach and the stability of the transition state, we can gain a deeper appreciation for the dramatic impact of steric hindrance on SN2 reactivity.
Video: SN2 Unreactive Alkyl Halides: The Shocking Truth!
FAQs: SN2 Unreactive Alkyl Halides
Got questions about why some alkyl halides just don't play ball in SN2 reactions? Here are some common queries and their answers:
Why are some alkyl halides considered "SN2 unreactive"?
SN2 reactions are all about the backside attack. Steric hindrance around the carbon bearing the leaving group severely hinders the nucleophile's approach. Therefore, alkyl halides with bulky groups attached to the carbon or adjacent carbons are essentially unreactive in an SN2 reaction.
What makes an alkyl halide sterically hindered?
Bulkier groups near the reaction center act as roadblocks. This means tertiary alkyl halides, and even some secondary ones with large substituents, become difficult (or impossible) for the nucleophile to attack. The crowding prevents the necessary transition state from forming.
Besides tertiary alkyl halides, what other structures might be SN2 unreactive?
Neopentyl halides are excellent examples. Even though the carbon bonded to the halogen isn't directly bonded to three other carbons, the three methyl groups on the neighboring carbon create significant steric hindrance. Similarly, any alkyl halide with bulky groups branched near the reactive carbon could exhibit this behavior. The more branched groups nearby, the slower the reaction.
Is it completely impossible for an SN2 reaction to occur with these unreactive alkyl halides?
Practically, yes. While theoretically possible under extreme conditions, the rate is so slow that other reactions will dominate. In most lab settings, you’d consider them unreactive. Thus, when asked, which of the alkyl halides shown is essentially unreactive in an sn2 reaction, these would be it.