Meso Compounds: Active or Inactive? The Optical Mystery!

Stereochemistry, a cornerstone of organic chemistry, significantly influences the properties of molecules. Chirality, an essential aspect of stereochemistry, describes molecules lacking a plane of symmetry, often leading to optical activity. Meso compounds, although possessing chiral centers, exhibit unique symmetry properties. The question of meso compounds are optically active or inactive becomes crucial in understanding their behavior. Polarimetry, a laboratory technique measuring the rotation of polarized light, helps determine a compound's optical activity, or lack thereof.
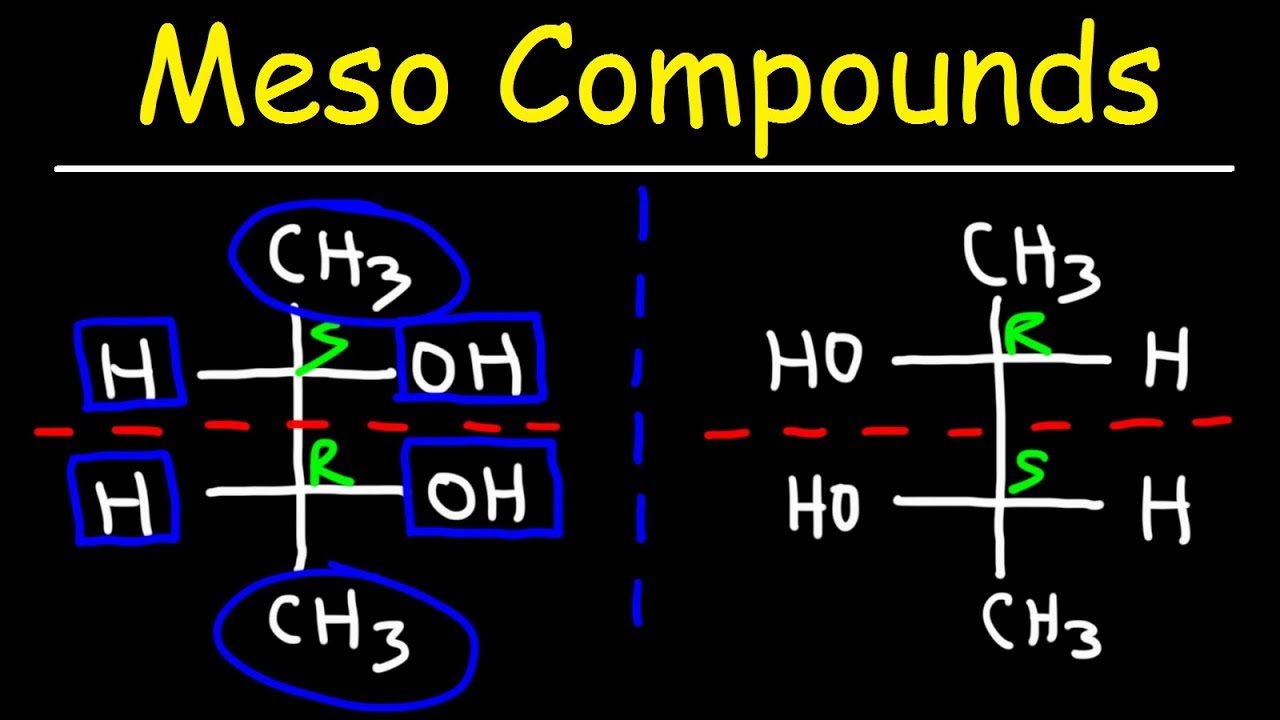
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Meso Compounds .
Have you ever wondered why some molecules, despite possessing seemingly "handed" structures, don't rotate plane-polarized light? It's a perplexing question that delves into the heart of stereochemistry and the fascinating phenomenon of optical activity.
This exploration isn't merely an academic exercise; understanding why some chiral molecules are optically inactive unlocks deeper insights into molecular structure and its impact on physical properties.
What is Optical Activity?
Optical activity refers to the ability of a chiral substance to rotate the plane of plane-polarized light.
When a beam of plane-polarized light passes through a solution containing an optically active compound, the plane of polarization is rotated either clockwise (dextrorotatory, designated as d or (+)) or counterclockwise (levorotatory, designated as l or (-)).
This rotation is a direct consequence of the molecule's asymmetry and its interaction with the electromagnetic field of light.
Chirality and the Expectation of Optical Activity
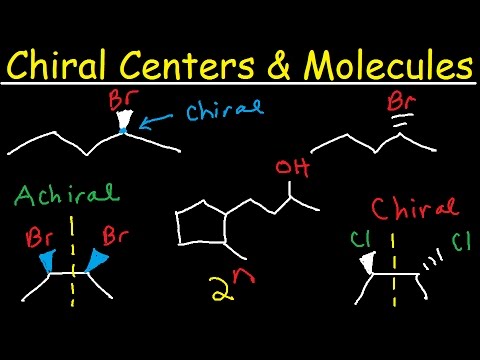
Generally, we expect that chirality, the property of a molecule being non-superimposable on its mirror image, leads to optical activity. A molecule is chiral if it lacks an internal plane of symmetry. The most common cause of chirality in organic molecules is the presence of a chiral center, which is typically a carbon atom bonded to four different groups.
However, this seemingly straightforward relationship encounters a fascinating exception.
The Meso Compound Exception: A Structural Anomaly
While chirality is a prerequisite for optical activity, it isn't always a guarantee. Enter meso compounds: molecules that possess chiral centers but exhibit no optical activity.
These compounds represent a fascinating exception to the rule, challenging our initial assumptions about the connection between molecular structure and optical properties.
The key to understanding this apparent contradiction lies in their unique structural feature: an internal plane of symmetry.

Therefore, in this article, we will be discussing that while chirality often leads to optical activity, meso compounds, despite possessing chiral centers, are an exception due to their unique structural properties.
Chirality and Stereoisomers: The Foundation
Before diving into the peculiar behavior of meso compounds, it's crucial to establish a firm understanding of the fundamental principles that govern their existence. We must first explore the concepts of chirality and stereoisomerism. These two ideas work in concert to provide the framework for understanding how molecules can have the same connectivity yet exhibit radically different properties.
Defining Chirality: Handedness in Molecules
Chirality, derived from the Greek word for "hand," refers to the property of a molecule being non-superimposable on its mirror image. Just like our left and right hands, chiral molecules are mirror images that cannot be perfectly overlaid onto one another. This handedness arises from the three-dimensional arrangement of atoms within the molecule.
The most common source of chirality in organic molecules is the presence of a chiral center.
A chiral center, also known as an asymmetric carbon, is typically a carbon atom bonded to four different substituents (atoms or groups of atoms). This tetrahedral arrangement creates a situation where no matter how the molecule is rotated, its mirror image will always be distinct.
Chirality and the Emergence of Stereoisomers
Chirality is the underlying cause of stereoisomerism.
Stereoisomers are molecules that have the same molecular formula and the same connectivity of atoms, but differ in the three-dimensional arrangement of their atoms in space.
In other words, they are isomers that are only different because their atoms are oriented differently.
This subtle difference in spatial arrangement can lead to significant differences in physical and chemical properties, most notably in their interaction with plane-polarized light.
Enantiomers: Mirror Images with Distinct Properties
Enantiomers are a special type of stereoisomer.
Enantiomers are stereoisomers that are non-superimposable mirror images of each other. They possess identical physical properties, such as melting point, boiling point, and refractive index, except for one crucial difference: their interaction with plane-polarized light.
One enantiomer will rotate plane-polarized light clockwise (dextrorotatory), while the other will rotate it counterclockwise (levorotatory) to the same degree.
This unique property is what defines them as optically active.
Diastereomers: Stereoisomers Beyond Mirror Images
Diastereomers represent another class of stereoisomers.
Diastereomers are stereoisomers that are not mirror images of each other. Unlike enantiomers, diastereomers can have different physical properties, such as melting point, boiling point, and solubility.
Diastereomers arise when a molecule has two or more chiral centers, and some, but not all, of those centers are inverted in the stereoisomer.
Chirality, then, sets the stage for stereoisomerism, dictating that molecules with the same connections can still differ profoundly. But not every molecule with chiral centers participates fully in this dance of optical activity. A curious exception exists, challenging the seemingly straightforward relationship between chirality and how a compound interacts with light. This leads us to meso compounds, unique structures that harbor chiral centers yet remain optically inactive due to their inherent symmetry.
Meso Compounds Defined: Structure and Symmetry
Meso compounds represent a fascinating intersection of chirality and symmetry in molecular architecture. They are molecules that contain chiral centers, a characteristic they share with many optically active compounds.
The Defining Characteristic: Internal Plane of Symmetry
What distinguishes meso compounds is the presence of an internal plane of symmetry. This plane effectively divides the molecule into two halves that are mirror images of each other.
This mirror-image relationship within the same molecule is key to understanding their optical inactivity.
Visualizing the Plane of Symmetry
Imagine bisecting a meso compound with a plane. On one side, you see a particular arrangement of substituents around a chiral center. On the other side, you see the exact mirror image of that arrangement.
This internal reflection effectively cancels out any potential optical activity.
A Simple Example: 2,3-Dichlorobutane
A classic example of a meso compound is 2,3-dichlorobutane. To recognize its meso form, draw the molecule in a conformation where the two chlorine atoms are positioned such that a plane of symmetry can be easily visualized bisecting the central carbon-carbon bond.
This plane of symmetry makes the molecule superimposable on its mirror image, rendering it achiral despite the presence of two chiral carbon atoms.
Implications of Symmetry
The existence of this internal mirror plane means that the molecule, as a whole, is not chiral, even though it possesses chiral centers. The symmetry overrides the chirality at each center.
Chirality, then, sets the stage for stereoisomerism, dictating that molecules with the same connections can still differ profoundly. But not every molecule with chiral centers participates fully in this dance of optical activity. A curious exception exists, challenging the seemingly straightforward relationship between chirality and how a compound interacts with light. This leads us to meso compounds, unique structures that harbor chiral centers yet remain optically inactive due to their inherent symmetry.
Internal Compensation: The Secret to Inactivity
The optical inactivity of meso compounds stems from a fascinating phenomenon called internal compensation. While chiral centers within the molecule possess the potential to rotate plane-polarized light, the molecule's inherent symmetry cleverly negates this effect. This section delves into the mechanism of internal compensation, revealing why meso compounds defy the conventional expectation of optical activity.
The Role of the Internal Plane of Symmetry
The internal plane of symmetry is the key to understanding internal compensation. This plane divides the molecule into two halves that are mirror images of each other. Each half contains at least one chiral center. Critically, these chiral centers, while stereocenters, exhibit opposite configurations.
This symmetrical arrangement has profound consequences for how the molecule interacts with plane-polarized light.
Equal and Opposite Rotation of Light
In essence, one half of the meso compound rotates plane-polarized light in a specific direction.
Simultaneously, the other half of the molecule, being its mirror image, rotates the light by an equal amount, but in the opposite direction.
This creates a "tug-of-war" effect where the two halves cancel each other out.
Net Optical Rotation of Zero
The result of this internal compensation is a net optical rotation of zero.
Even though the molecule possesses chiral centers capable of rotating light, the overall effect is that the light passes through the compound without any change in its plane of polarization.
This is why meso compounds are classified as optically inactive, despite their chiral nature.
Internal compensation provides a compelling explanation for the optical inactivity observed in meso compounds. But how do we experimentally verify whether a substance is, in fact, optically active? The answer lies in a specialized instrument called the polarimeter.
The Polarimeter: Unveiling Optical Activity Through Light
The polarimeter is an instrument designed to measure the optical activity of a substance. It allows us to quantitatively determine the extent to which a compound rotates plane-polarized light, providing crucial insights into its stereochemical properties.
How a Polarimeter Works: A Step-by-Step Look
A polarimeter works by passing a beam of plane-polarized light through a sample and then measuring the angle by which the light's plane of polarization has been rotated.
-
Light Source and Polarizer: The process begins with a light source, typically a sodium lamp, emitting light of a specific wavelength. This light passes through a polarizer, which filters the light, allowing only light waves vibrating in a single plane to pass through. This resulting light is known as plane-polarized light.
-
Sample Cell: The plane-polarized light then travels through a sample cell containing the substance being analyzed. If the substance is optically active, it will interact with the plane-polarized light and rotate its plane of polarization.
-
Analyzer and Detector: After passing through the sample, the light encounters an analyzer, another polarizer. The analyzer is rotated until it allows the maximum amount of light to pass through, indicating that it is aligned with the rotated plane of polarization. The angle of rotation required to achieve this alignment is then measured by a detector.
The Role of Plane-Polarized Light
Plane-polarized light is the key to the polarimeter's function. Normal light vibrates in all directions perpendicular to its direction of travel. When light is polarized, it is forced to vibrate in a single plane. This controlled orientation allows chiral molecules to interact with the light in a specific way, leading to rotation.
Meso Compounds and the Polarimeter: No Rotation Observed
A crucial observation arises when examining meso compounds with a polarimeter: they exhibit no net rotation of plane-polarized light. Despite possessing chiral centers, the internal compensation within the molecule cancels out any potential rotation. The polarimeter, therefore, provides experimental confirmation of the theoretical concept of internal compensation.
In practical terms, this means that when a meso compound is placed in the sample cell of a polarimeter, the analyzer will not need to be rotated to achieve maximum light transmission. The measured rotation will be zero, indicating the absence of optical activity. This characteristic behavior in the polarimeter serves as a definitive test for identifying meso compounds.
Internal compensation provides a compelling explanation for the optical inactivity observed in meso compounds. But how do we experimentally verify whether a substance is, in fact, optically active? The answer lies in a specialized instrument called the polarimeter.
With a grasp of how a polarimeter confirms the absence of optical activity in meso compounds, it's time to place these unique molecules within the broader context of stereoisomers. How do meso compounds stack up against their stereoisomeric cousins, enantiomers and diastereomers? The answer lies in their structural differences and symmetry.
Meso vs. Other Stereoisomers: A Comparative Analysis
Understanding the distinction between meso compounds, enantiomers, and diastereomers is crucial in stereochemistry. While all three are stereoisomers (molecules with the same connectivity but different spatial arrangements), they exhibit significant differences in their structures, symmetry, and, most notably, their optical activity.
Structural Distinctions and Symmetry
The defining characteristic of a meso compound is the presence of chiral centers alongside an internal plane of symmetry. This internal symmetry is the key differentiating factor.
Enantiomers, on the other hand, are stereoisomers that are non-superimposable mirror images of each other. They possess chiral centers but lack any internal plane of symmetry. This absence of symmetry is the root cause of their optical activity.
Diastereomers are stereoisomers that are not mirror images of each other. They may or may not possess chiral centers and can have varying degrees of structural similarity. Crucially, they also lack the specific internal symmetry found in meso compounds.
Optical Activity: The Decisive Difference
The structural differences directly impact the optical activity of these stereoisomers.
Enantiomers are always optically active. Each enantiomer rotates plane-polarized light to an equal extent but in opposite directions (+ and -).
Meso compounds, as we've established, are optically inactive due to internal compensation.
Diastereomers can be either optically active or inactive, depending on their specific structure. The absence of internal symmetry doesn't guarantee optical activity, but it does permit it. The key consideration for diastereomers is whether the molecule as a whole is chiral.
The Role of the Internal Plane of Symmetry
The presence or absence of an internal plane of symmetry is the most reliable indicator for differentiating meso compounds from other stereoisomers.
If a molecule with chiral centers possesses such a plane, it is a meso compound and optically inactive.
Conversely, if a molecule with chiral centers lacks this plane, it is likely an enantiomer or a diastereomer and, therefore, potentially optically active. It is the absence that allows the free rotation.
By carefully examining the structure and identifying any internal symmetry, chemists can accurately classify stereoisomers and predict their optical properties.
With a firm grasp of the structural and symmetrical properties that dictate optical activity, it's time to move beyond qualitative descriptions and delve into the quantitative measurement of this fascinating phenomenon. While we can visually identify the presence or absence of optical activity using a polarimeter, how can we precisely quantify the degree to which a substance rotates plane-polarized light? This is where the concept of specific rotation comes into play, offering a more refined and informative approach to analyzing optical properties.
Understanding Specific Rotation: A Quantitative Approach
Specific rotation provides a standardized way to compare the optical activity of different compounds. It moves beyond simply stating whether a substance is optically active or inactive.
Instead, it allows us to express the magnitude of optical rotation in a way that accounts for factors like concentration and path length.
Defining Specific Rotation
Specific rotation, denoted by the symbol [α], is defined as the observed rotation of plane-polarized light by a chiral compound under specific and standardized conditions.
The defining formula is:
[α] = α / (l * c)
Where:
- [α] is the specific rotation
- α is the observed rotation in degrees
- l is the path length of the sample cell in decimeters (dm)
- c is the concentration of the sample in grams per milliliter (g/mL)
These conditions are crucial. By controlling the wavelength of light, temperature, concentration, and path length, we can obtain a reproducible value for specific rotation.
Standard Conditions for Measurement
The standard conditions are usually at a temperature of 20°C, using the sodium D line (589 nm) as the light source. This is represented as [α]D20.
These controlled parameters ensure that the specific rotation value is consistent and comparable across different laboratories and experiments.
How Specific Rotation Differentiates Stereoisomers
Specific rotation is a powerful tool for differentiating between stereoisomers, particularly enantiomers.
Enantiomers have equal but opposite specific rotations. If one enantiomer rotates plane-polarized light +X°, its mirror image will rotate the light -X°.
This provides a clear and unambiguous way to distinguish between these mirror-image isomers.
Diastereomers, on the other hand, will have different specific rotations that are unrelated in magnitude or sign.
The specific rotation of a diastereomer is an independent property of that specific compound.
This difference allows us to not only identify but also characterize complex mixtures of stereoisomers.
Applications of Specific Rotation
Specific rotation has broad applications in various fields of chemistry, including:
- Pharmaceutical Chemistry: Assessing the purity and identity of chiral drugs.
- Natural Product Chemistry: Characterizing and identifying new chiral compounds isolated from natural sources.
- Organic Synthesis: Monitoring the stereochemical outcome of chemical reactions.
- Food Chemistry: Analyzing the composition and authenticity of food products.
In essence, specific rotation is a quantitative fingerprint for chiral molecules, offering a detailed and reliable way to study their optical properties.
Video: Meso Compounds: Active or Inactive? The Optical Mystery!
Meso Compounds: Unraveling the Optical Mystery - FAQs
Here are some frequently asked questions to help clarify the concept of meso compounds and their optical activity.
What exactly defines a meso compound?
A meso compound is a molecule that contains chiral centers but is achiral overall. This is due to the presence of an internal plane of symmetry or a center of inversion within the molecule. The symmetry cancels out the optical activity expected from the chiral centers.
Why are meso compounds optically inactive despite having chiral centers?
Because of the internal symmetry, one half of the molecule rotates plane-polarized light in one direction, while the other half rotates it in the opposite direction. This equal and opposite rotation results in a net rotation of zero. Therefore, meso compounds are optically active or inactive.
How can I identify a meso compound?
Look for chiral centers within the molecule and then examine the structure for an internal plane of symmetry or a center of inversion. If either of these symmetry elements is present, and there are chiral centers, the compound is likely meso. Visualizing a 3D model can also be helpful.
Does the presence of multiple chiral centers always guarantee optical activity?
No. While chiral centers are a prerequisite for optical activity, the overall molecule can still be achiral if it possesses a meso configuration. The internal symmetry nullifies the effect of the individual chiral centers, and meso compounds are optically active or inactive.
Hopefully, that clears up some of the mystery surrounding meso compounds are optically active or inactive! It can be a tricky concept, but with a little practice, it'll become second nature. Keep exploring, and let your curiosity lead you!