Demystifying Atomic Mass: Is It Really an Average?

Understanding atomic mass is foundational to comprehending chemistry, and the average atomic mass of an element is the average of the atomic masses of its isotopes. Isotopes, variations of an element with differing neutron counts, contribute to this average. The International Union of Pure and Applied Chemistry (IUPAC), a globally recognized authority, provides standardized values for atomic weights, reflecting the relative abundance of these isotopes. Scientists utilize mass spectrometry, a powerful analytical technique, to precisely determine isotopic masses and abundances, allowing for accurate calculation of average atomic mass. This process ultimately helps professionals working with periodic table accurately predict the behavior of elements in chemical reactions.
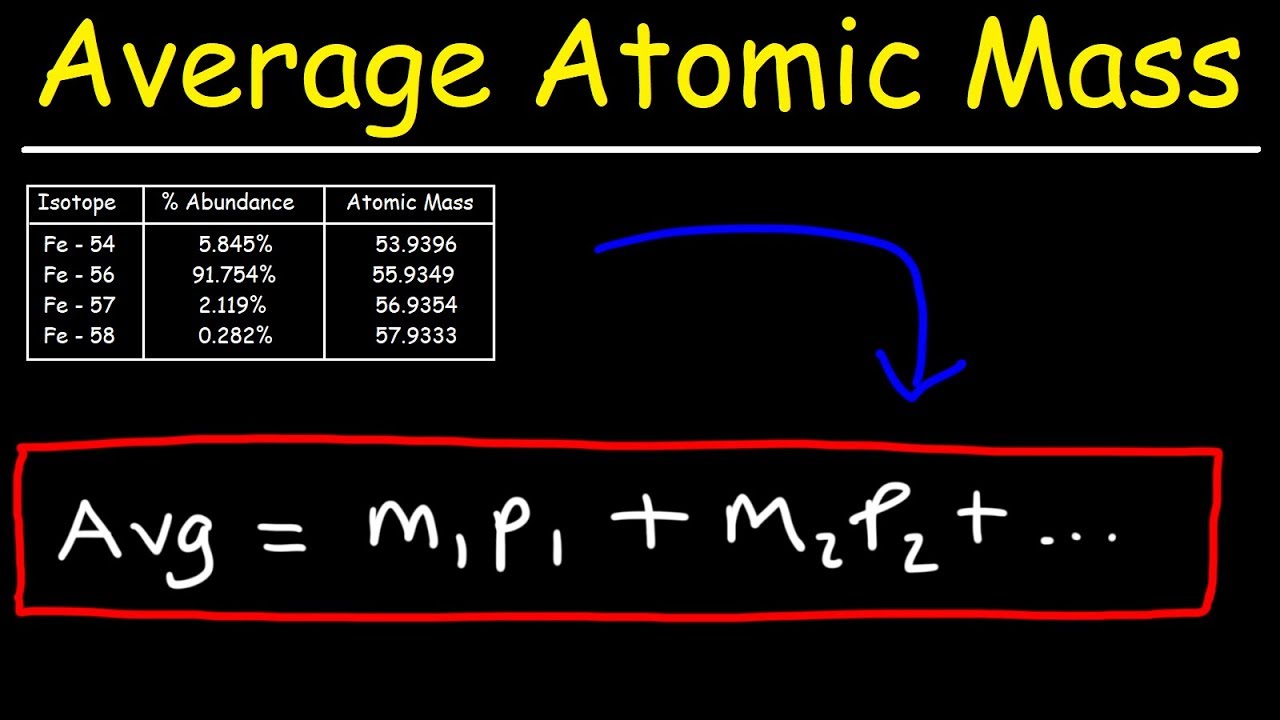
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled How To Calculate The Average Atomic Mass .
Demystifying Atomic Mass: Is It Really an Average?
Atomic mass can be a tricky concept to grasp. While often described as an "average," it's crucial to understand precisely what is being averaged and why. This explanation will break down the components of atomic mass and clarify how the average is calculated. The core principle lies in recognizing that the average atomic mass of an element is the average of the atomic masses of its naturally occurring isotopes, weighted by their relative abundance.
Understanding the Building Blocks: Atoms and Isotopes
Atoms: The Foundation
Atoms are the basic units of matter, composed of protons, neutrons, and electrons. The number of protons defines the element; for example, all atoms with one proton are hydrogen. The number of neutrons, however, can vary.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element that have different numbers of neutrons. Because neutrons contribute to the mass of an atom, different isotopes of the same element have different masses. For example, carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C) are all isotopes of carbon. They all have six protons, but ¹²C has six neutrons, ¹³C has seven neutrons, and ¹⁴C has eight neutrons.
Atomic Mass vs. Mass Number
It’s important to differentiate between atomic mass and mass number.
-
Mass Number: The mass number represents the total number of protons and neutrons in an atom's nucleus. It's always a whole number.
-
Atomic Mass: Atomic mass is the actual mass of an atom, usually expressed in atomic mass units (amu). It is very close to but not equal to the mass number and accounts for the mass deficit due to binding energy. Atomic mass is a measured value.
The "Average" in Atomic Mass: Weighted Averages and Isotopic Abundance
The term "average atomic mass" refers to a weighted average. This is where the average atomic mass of an element is the average of the atomic masses of its isotopes, but each isotope's contribution is proportional to how abundant it is in nature.
Isotopic Abundance: How Much of Each?
Isotopic abundance refers to the percentage of each isotope found naturally. These abundances are typically determined experimentally and are relatively consistent across different samples of the same element. For example, a sample of carbon will consistently contain approximately 98.9% ¹²C and 1.1% ¹³C, with trace amounts of ¹⁴C.
Weighted Average Calculation: Putting it All Together
To calculate the average atomic mass, you need two key pieces of information:
- The atomic mass of each isotope.
- The relative abundance of each isotope (expressed as a decimal).
The calculation is as follows:
(Atomic Mass of Isotope 1 × Abundance of Isotope 1) + (Atomic Mass of Isotope 2 × Abundance of Isotope 2) + ...
Let's illustrate this with an example: chlorine (Cl), which has two naturally occurring isotopes, Chlorine-35 (³⁵Cl) and Chlorine-37 (³⁷Cl).

- ³⁵Cl: Atomic Mass = 34.969 amu, Abundance = 75.77% (0.7577)
- ³⁷Cl: Atomic Mass = 36.966 amu, Abundance = 24.23% (0.2423)
Average Atomic Mass of Chlorine = (34.969 amu × 0.7577) + (36.966 amu × 0.2423) = 26.495 amu + 8.957 amu = 35.452 amu
Therefore, the average atomic mass of chlorine is approximately 35.45 amu. This is the value you would find on the periodic table. This reflects that the average atomic mass of an element is the average of the atomic masses of its various isotopes, accounting for how frequently each isotope is found in nature.
Why Use Weighted Averages?
The periodic table lists atomic masses, not the mass of any single atom. Since elements exist as mixtures of isotopes, a weighted average provides a useful and representative value for calculations in chemistry. It allows chemists to work with macroscopic quantities of elements without having to consider the individual isotopic composition of each atom.
Example: Calculating Average Atomic Mass of Copper
Let's look at another example, Copper (Cu), which has two stable isotopes:
| Isotope | Atomic Mass (amu) | Relative Abundance (%) |
|---|---|---|
| Copper-63 (⁶³Cu) | 62.9296 | 69.15 |
| Copper-65 (⁶⁵Cu) | 64.9278 | 30.85 |
To calculate the average atomic mass:
- Convert the percentage abundances to decimal form by dividing by 100.
- 69.15% becomes 0.6915
- 30.85% becomes 0.3085
- Multiply the atomic mass of each isotope by its decimal abundance.
- (62.9296 amu * 0.6915) = 43.514 amu
- (64.9278 amu * 0.3085) = 20.029 amu
- Add the results together to get the average atomic mass.
- 43.514 amu + 20.029 amu = 63.543 amu
Therefore, the average atomic mass of copper is approximately 63.54 amu, illustrating again that the average atomic mass of an element is the average of the atomic masses of its naturally occurring isotopes, considering their proportional abundance.
