Copper's Secret Code: Unlocking Its Atomic Number!

The element Copper, a staple in fields like Electrical Engineering and celebrated in places such as Chuquicamata (a major copper mine), holds a fascinating position on the periodic table. Its properties, studied extensively by organizations like IUPAC, are crucial for understanding its myriad applications. So, if you've ever wondered what is the atomic number of copper and why it matters, prepare to decode this metal's elemental fingerprint with us.

Image taken from the YouTube channel Notes Reel , from the video titled What is the atomic number of copper? .
Unlocking Copper's Atomic Identity
Copper. It's a metal we encounter daily, often without a second thought.
From the intricate wiring that powers our homes to the durable pipes that deliver our water, copper is a silent workhorse of modern society.
Its reddish-gold hue is instantly recognizable, yet few truly understand the fundamental characteristic that defines this element: its atomic number.
Imagine each element possessing a secret code, a unique identifier that dictates its properties and behavior. This "secret code" is the atomic number.
In this article, we embark on a journey to decipher this code for copper.
Copper: The Indispensable Metal
Copper (Cu), derived from the Latin cuprum, has been essential to human civilization for millennia.
Its exceptional electrical and thermal conductivity, coupled with its malleability and resistance to corrosion, make it invaluable across a spectrum of applications.
Consider the electrical grid that powers our cities. Copper wiring is the backbone of this network, efficiently transmitting electricity over vast distances.
Likewise, in plumbing systems, copper pipes provide a safe and reliable means of transporting water, resisting the corrosive effects that plague other materials.
Beyond these common uses, copper finds its way into countless alloys, such as brass and bronze, enhancing the strength and durability of everything from musical instruments to marine hardware.

The Atomic Number: Copper's Defining Trait
But what truly sets copper apart? What makes it copper and not gold, silver, or iron?
The answer lies in its atomic number, a fundamental property that governs its very essence.
It's more than just a number; it's the key to understanding copper's behavior, its interactions with other elements, and its remarkable utility.
This article aims to illuminate this concept.
Our goal is to explain precisely what the atomic number of copper is, how it defines copper, and why this seemingly simple number holds such profound significance.
Join us as we delve into the heart of matter and unravel the secrets of copper's atomic identity.
Atomic Number: The Element's Fingerprint
We've established that copper's unique qualities make it an indispensable material. But what truly dictates those qualities, separating it from the myriad of other elements in the universe? The answer lies in a single, powerful number: the atomic number.
Decoding the Atomic Number
The atomic number is more than just a label; it's the fundamental identifier of an element. Think of it as the element's unique fingerprint, its permanent and unchangeable signature.
It defines what that element is and dictates how it interacts with other elements.
The Proton Connection
So, what exactly is the atomic number, and what does it represent? At its core, the atomic number defines the number of protons found within the nucleus of an atom of that element.
Protons are positively charged particles residing in the atom's nucleus. The number of these protons is the defining characteristic of the element.
For instance, every atom with one proton is hydrogen. Every atom with two protons is helium. Change the number of protons, and you change the element itself.
This seemingly simple concept has profound implications for understanding the nature of matter.
Navigating the Periodic Table
The periodic table is chemistry's most essential cheat sheet. This chart organizes all known elements based on their atomic number and recurring chemical properties.
It's where you can quickly discover an element's atomic number. Elements are arranged in ascending order of their atomic number, starting with hydrogen (atomic number 1) and continuing sequentially across the table.
You can find the atomic number displayed as a whole number above the element's symbol. This organization allows chemists and scientists to easily predict and understand the properties and behavior of different elements.
Copper Revealed: Decoding its Atomic Number
Having explored the significance of atomic numbers in general, let's now turn our attention to copper and unveil its specific atomic identity. This understanding is crucial for appreciating copper's unique properties and behavior.
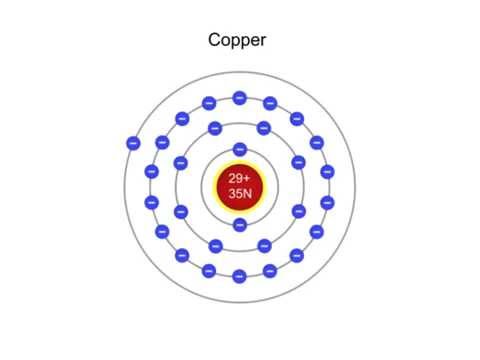
Unveiling Copper's Identity: The Atomic Number 29
The atomic number of copper (Cu) is 29. This seemingly simple number is a profound statement about the very nature of this element.
It is the key to understanding why copper behaves the way it does.
The Significance of 29: Protons at the Core
What does the atomic number 29 actually tell us about copper?
It tells us that every atom of copper contains exactly 29 protons within its nucleus. This is not an approximation; it's an absolute and defining characteristic.
Change that number, and you no longer have copper.
You have a different element altogether. The number of protons is what fundamentally defines an element's identity.
Protons and Electrons: Maintaining Neutrality
In a neutral copper atom, the number of protons is balanced by an equal number of electrons orbiting the nucleus.
Electrons are negatively charged particles.
Therefore, a neutral copper atom, with its 29 positively charged protons, will also have 29 negatively charged electrons.
This balance of charge is what keeps the atom electrically neutral. It's this arrangement of electrons that ultimately dictates how copper interacts with other elements, forming compounds and participating in chemical reactions.
Having established that copper possesses 29 protons, and consequently 29 electrons in its neutral state, the question naturally arises: where does copper reside within the grand scheme of elements, and how does its placement relate to its inherent characteristics?
Copper's Periodic Home and Intrinsic Properties
Copper's Address: Group 11, Period 4
The Periodic Table of Elements is not just a list; it's a carefully organized map reflecting the relationships between elements.
Copper (Cu) proudly resides in Group 11 (also known as the coinage metals) and Period 4.
This location isn't arbitrary; it's a direct consequence of its electron configuration.
Elements in the same group share similar chemical properties due to having the same number of valence electrons (electrons in the outermost shell).
Copper, along with silver and gold, exhibits characteristics of transition metals.
Properties of Copper: Conductivity and Malleability
Copper's position on the periodic table offers clues to its most notable characteristics.
Its electronic structure contributes directly to its outstanding conductivity, both electrical and thermal.
The loosely held valence electrons can move freely, facilitating the flow of charge or heat.
Furthermore, copper is exceptionally malleable and ductile, meaning it can be hammered into thin sheets or drawn into wires without breaking.
These properties make it invaluable in electrical wiring, plumbing, and various manufacturing processes.
The Role of Electron Configuration
The arrangement of electrons in copper atoms is crucial for these properties.
Specifically, copper exhibits an exception to the typical filling order of electron orbitals.
This unique configuration contributes to the stability of the atom and enhances its conductive capabilities.
Isotopes: Variations on a Theme
While all copper atoms share the same atomic number (29), they can differ in their number of neutrons.
Atoms of the same element with different numbers of neutrons are called isotopes.
Copper has two stable isotopes: copper-63 (²⁹Cu) and copper-65 (²⁹Cu).
Copper-63 has 34 neutrons (63 - 29 = 34), while copper-65 has 36 neutrons.
These isotopes exhibit slightly different masses but share virtually identical chemical properties.
The existence of isotopes doesn't change copper's fundamental identity.
The atomic number, the number of protons, remains the defining characteristic.
Having established that copper possesses 29 protons, and consequently 29 electrons in its neutral state, the question naturally arises: where does copper reside within the grand scheme of elements, and how does its placement relate to its inherent characteristics?
The Power of 29: Atomic Number and Copper's Behavior
Copper's atomic number, 29, is more than just a label; it's the fundamental determinant of its chemical behavior. This single number dictates how copper interacts with other elements, forming compounds and participating in chemical reactions. It's the key to understanding why copper is so uniquely suited to its myriad applications.
Atomic Number as the Blueprint for Chemical Behavior
The atomic number's influence extends to every facet of copper's chemistry. Because the number of protons dictates the number of electrons (in a neutral atom), it defines the element's electron configuration.
This configuration, in turn, governs how an atom interacts with other atoms. Copper's tendency to form certain types of bonds, its preferred oxidation states, and its reactivity with acids or bases are all direct consequences of having 29 protons.
Electron Configuration: The Architectural Plan
Electron configuration describes the arrangement of electrons within an atom's energy levels and orbitals. This arrangement dictates how an atom will interact with other atoms. For copper, its electron configuration is somewhat unusual.
Instead of completely filling the 4s orbital before adding electrons to the 3d orbital, one electron from the 4s orbital jumps to the 3d orbital, resulting in a completely filled 3d orbital and a half-filled 4s orbital. This is written as [Ar] 3d¹⁰ 4s¹.
This seemingly minor detail has profound implications. This unique configuration makes copper a relatively stable metal, contributing to its resistance to corrosion.
It also plays a crucial role in its exceptional electrical conductivity.
The Number 29 and Copper's Versatile Applications
The properties dictated by copper's atomic number translate directly into its widespread use across diverse industries.
Its high electrical conductivity, a consequence of its electron configuration, makes it the ideal material for electrical wiring and circuitry. The free movement of electrons allows for efficient transmission of electricity.
Its malleability and ductility, also related to its metallic bonding characteristics, allow it to be easily drawn into wires and shaped into various components.
In plumbing, copper's resistance to corrosion and its ability to inhibit bacterial growth make it a safe and reliable choice for water pipes.
Copper's ability to form alloys with other metals expands its utility even further. Brass (copper and zinc) and bronze (copper and tin) are stronger and more durable than pure copper, making them suitable for applications ranging from musical instruments to marine hardware.
The specific properties conferred by the atomic number 29 explain its versatility and significance in countless modern technologies and industries. From conducting electricity to resisting corrosion, the number 29 is the foundation upon which copper's utility is built.
Video: Copper's Secret Code: Unlocking Its Atomic Number!
FAQs: Cracking Copper's Atomic Number Code
[Opening Paragraph] We've explored copper's atomic number. These frequently asked questions will help solidify your understanding of this key element property.
What exactly does "atomic number" mean?
The atomic number represents the number of protons found in the nucleus of an atom. It's a fundamental property that defines what element an atom is. For instance, all atoms with 29 protons are copper atoms.
How does knowing the atomic number help us identify copper?
The atomic number is like a unique identification code for each element. Since the atomic number of copper is 29, any atom with 29 protons must be a copper atom. It's a definitive identifier.
What is the atomic number of copper and why is it important?
The atomic number of copper is 29. This means that every copper atom has 29 protons in its nucleus. This number is essential because it dictates copper's chemical behavior and how it interacts with other elements.
Where can I find the atomic number of copper listed?
You can find the atomic number of copper (29) in the periodic table of elements. It's typically displayed near the element's symbol (Cu). Most science textbooks and reliable online resources will also provide this information.